Abstract
Plant-mediated fabrication of nanomaterials has gained remarkable recognition due to its environmental friendliness and economic efficiency. In the present study, a green synthesis protocol, based on Pulicaria jaubertii leaves decoction, was employed to manufacture silver nanoparticles (AgNPs). The constituents of the leaf extract served a two-fold role, first as an agent to reduce ionic silver to silver nanoparticles (AgNPs); secondly by stabilizing the as-synthesized AgNPs by capping their surface. The as-fabricated silver nanoparticles (AgNPs) were scrutinized for biophysical characterization using UV-VIS spectroscopy, dynamic light scattering (DLS), Fourier transform infrared (FT-IR) spectroscopy, transmission electron microscopy (TEM), scanning electron microscopy (SEM), and energy-dispersive X-ray (EDX) spectroscopy. Next, we explored the anti-bacterial activity of AgNPs against two micro-organisms namely Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus). We have also deciphered the antibiofilm potential of the as-fabricated AgNPs against E. coli and S. aureus. Additionally, the as-fabricated AgNPs manifested the least toxicity to peripheral blood mononuclear cells (PBMC) and human red blood cells (RBCs), signifying that as-synthesized nanoparticles are innocuous to be brought into use as future medicinal agents.
Keywords
Silver nanoparticles, Pulicaria jaubertii, Green synthesis, Cytotoxic, Microbes, Antimicrobial
Introduction
Nanomaterials, such as silver nanoparticles, have been researched intensely across the world. The average size of the metal-based nanoparticles including AgNPs has been reported to be less than 100 nm. In general, nanoparticles, compared with their large bulk precursor material, exhibit reformed or enhanced properties, in terms of their characteristic features including morphology, dimensions, size distribution, etc [1]. Nanomaterials have entertained considerable attention for their special biological, chemical, physical, and optical properties. They have been widely exploited in multiple fields including biomedicine, drug delivery, agriculture, water treatment, topical ointments, cosmetics, electronics, textile industry, etc [2,3]. Nanoparticles can be synthesized through biological, physical, and chemical approaches. Nanoparticle synthesis using the chemical method is advantageous because it requires less time for the generation of large numbers of nanoparticles, however, this method is full of hazardous issues associated with the involved chemicals. Moreover, it fails to provide capping factors that can help in the stabilization of as-synthesized particles [4]. Further, the nanoparticles which are synthesized by the chemical method are incongruous for biological activities due to their toxicity [5,6]. The biological synthesis of nanoparticles using eco-friendly methods has invited significant attention to protect the environment from hazardous by-products of the nanoparticle fabrication process [7]. The biological materials such as the extract of fungi, bacteria, and algae on one hand and various types of plants, on the other, have been extensively employed in the green synthesis of a variety of nanoparticle manufacturing processes [8,9]. The ‘filtered plant extract’, upon its incubation with the solution of a metal-salt (cf. AgNO3) at a temperature of 25°C can mediate the nanoparticle synthesis. The progress of the synthesis can be followed on the basis of the changes in the physicochemical properties such as the colour change of the reaction mixture within a few minutes to several hours. The green synthesis method has remained stupendous in the synthesis of silver, gold, and other metallic nanoparticles [10]. The parameters such as character and potency of the plant extract as well as the concentration of the metal salt, pH, and temperature have a great deal of impact on the kinetics of nanoparticle synthesis. These factors also affect other physicochemical properties of the as-fabricated nanoparticles [11].
The plant Pulicaria genus belongs to Asteraceae family of plants and includes more than 100 species widespread around the world. Locally known as (Anssif), the plant is indigenous to Yemen and has been widely used in traditional medicine asan antipyretic, and diuretic. The leaves of this plant are used as a flavoring agent. The plant is also found in various other parts of Asia, Europe, and North Africa and is known for its odoriferous nature. Several properties, including anti-leukemic, anti-inflammatory, and cytotoxic activity, have been associated with Pulicaria species [12]. Some researchers have reported that the plant has antioxidant, antimicrobial, antimalarial, and insecticidal properties as well [13].
The primary goal of the present study was to generate AgNPs using Pulicaria jaubertii leaf extract. The method avoided the involvement of high heat input in the synthesis or generation of toxic by-products formed during the chemical synthesis approach. The aqueous extract of Pulicaria jaubertii\dried leaf powder was used to synthesize AgNPs. Finally, the as-fabricated AgNPs were tested for their antimicrobial efficacy against bacterial microbes namely E. coli and S. aureus. In addition, toxicity studies of as-fabricated AgNPs were carried out on human erythrocytes (RBCs) and peripheral blood mononuclear cells (PBMCs).
Materials and Methods
Materials
Chemicals such as silver nitrate salt (AgNO3) and MTT [3-(4,5-dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bromide] were procured from Merck, India. Agar powder, Luria Broth (LB), Brain Heart Infusion (BHI), and Muller-Hinton agar (MHA) were provided by Hi-media-India. FITC dye was procured from Sigma-Aldrich. The bacterial strains were a kind from Prof. Mohammad Shahid, provided by JNMC, Aligarh Muslim University (AMU), Aligarh, India.
Plant Collection and Preparation of the Leaf Extract
The Pulicaria jaubertii leaves were collected on 3rd September 2018 at Shameer district (one of the villages surrounding Taiz city, the Republic of Yemen). In the extraction process, the green leaves of Pulicaria jaubertii were washed 2-3 times with plain water and dried in a shadowed area. The dried leaves were pulverized to get a fine powder. Subsequently, about 10 grams of leaf powder was poured into an Erlenmeyer flask (500 ml) with 300 ml of distilled water and boiled till the volume gets halved. Whatman filter paper (pore size 0.45 microns) was used to filter the extract that was kept at 4°C for subsequent use. The aqueous leaf extract was used for the green synthesis of AgNPs [14].
AgNPs Green Synthesis
AgNPs were manufactured using a previously published procedure that was further standardized in our lab [15]. To execute the formation of metallic nanoparticles, Pulicaria jaubertii leaf extract was used as a reducing agent to convert the ionic state of Ag+ to Ag0. The leaf extract simultaneously functioned as a stabilizing and capping agent for the as-formed nanoparticles. Briefly, the aqueous solution of AgNO3 was prepared as a stock and used to synthesize AgNPs at a concentration of (10 mM). A fixed volume (500 μl) of Pulicaria jaubertii leaf extract was mixed with 2.5 ml silver nitrate solution (10 mM), and the volume was made to 5 ml by using distilled water. The solution was incubated for an extended time period in an incubator shaker at room temperature (25 ℃). A color change was witnessed wherein the light yellow color of reactant AgNO3 gets intensified to form a dark brown shade which implied that Ag+ of AgNO3 was being reduced to metallic silver (Ag0) [16].
Characterization Studies of As-fabricated AgNPs
UV-VIS Absorption Spectroscopy
UV-VIS double beam spectrophotometer was used for the identification, characterization, and analysis of metallic nanoparticles. The colored product was scanned in a range of wavelengths (300-700 nm) to follow the synthesis of as-fabricated AgNPs by using a UV-VIS spectrophotometer (Perkin-Elmer spectrophotometer) [17].
FTIR Spectroscopy Studies
Shimadzu IR Affinity-1 Fourier transforms infra-red spectrometer (IRAffinity-1, Japan), was used to analyze the functional groups of organic and inorganic compounds, in Pulicaria jaubertii leaf extract, the factors responsible for the reduction and stability of the as-formed AgNPs. The spectra were obtained by taking readings in the range of (4000 cm-1 to 400 cm-1) wavelength. As-synthesized AgNPs were utilized in the co-preparation of KBr crystals, which served as a beam splitter.
DLS and Zeta-potential Determination
DLS was used to assess the size distribution and average size of the as-fabricated AgNPs using the Malvern Zetasizer Nano-ZS90 (ZEN3590, UK). After passing the solution through a 0.22 m filter (Millipore), the nanoparticle suspension was lyophilized to get a powder. The powder form of the NPs was suspended in PBS and characterized to investigate the size and dispersal pattern of bio-fabricated AgNPs. The zeta potential of as-fabricated AgNPs was deciphered by the same instrument (ZEN3590, UK). The electrophoretic mobility (EPM) of nanoparticles was determined by employing (gel electrophoresis) after the samples were reconstituted in a buffer solution (5 mM PBS, pH 7.3). The AgNPs samples were analyzed in triplicate.
Electron Microscopic Studies
The surface properties of the as-fabricated AgNPs were characterized in terms of size, dimension, and surface morphology by TEM and SEM analysis. The TEM grid was made by adding one drop of the bio-reduced diluted solution to a copper grid that had been covered with carbon and dried under light (Dynopro-Tc-04 instrument protein solution). The surface morphology studies of as-synthesized AgNPs were carried out using TEM (JEOL model JSM67500F). We have also used Energy Dispersive X-Ray Analyzer for examining the elemental composition of the as-fabricated AgNPs.
Antibacterial Potential of As-fabricated AgNPs
Bacterial Strains
Antibacterial efficiency of as-fabricated AgNPs fabricated by using Pulicaria jaubertii leaf extract was tested on two common microbes, gram-positive, S. aureus and gram-negative, E. coli.
MIC of As-fabricated AgNPs against Bacterial Pathogens
MIC is the minimum concentration of a drug (antimicrobial agent) needed to inhibit obvious microbial growth. The MIC value of the as-manufactured AgNPs was evaluated using the broth microdilution procedure. Briefly, 100 μl of fresh media was added into the wells of a round-bottomed 96-well microtiter plate. Subsequently, 100 μl as-fabricated AgNPs (from a stock of 1 mg/ml) was added to the 1st well and serially diluted up to the last well. Subsequently, 100 μl of stock of each bacterial culture i.e., E. coli and S. aureus having106 CFU/ml was added into each well of 96 well micro-dilution culture plates. The wells that contained both medium and bacterial culture functioned as positive controls, whereas the wells that contained only media served as negative controls. Next, the microtiter plate was incubated for 24 hrs at 37°C. The bacterial growth was evaluated based on the turbidity of the broth, while the potential antimicrobial susceptibility was indicated by the decrease in the magnitude of turbidity of the broth. The MIC of AgNPs that can effectively inhibit bacterial growth was indicated by the lowest concentration where the broth was clear.
Assessing the Antibacterial Potential of AgNPs Using Agar Well Diffusion
The well diffusion assay was employed for the assessment of the antibacterial effect of as-fabricated AgNPs [18]. The bacterial test organisms were cultured overnight in fresh sterile LB broth (E. coli) and BHI broth (S. aureus) to achieve a colony-forming unit (CFU)of 106 per ml. A fixed volume (100 μl) of each bacterial culture was uniformly spread on the agar plate with the help of a plastic spreader. Wells were punched on agar plates. Varying concentration of AgNPs and gentamycin (as a standard antibiotic) using 1 mg/ml of stock solution were dispensed into different wells. The antibacterial potential of a combination of AgNPs and antibiotics was evaluated against standard microbes. The agar plates were incubated for 18 hrs at 37°C. The zone of inhibition was deciphered by determining the amount of clear zone that surrounded each well.
Time-kill Kinetic Studies
The as-fabricated AgNPs showing antibacterial activity were also assessed for their antimicrobial potential by employing time-kill kinetic studies. Both the bacterial strains namely, Escherichia coli and Staphylococcus aureus were cultured overnight at a temperature of 37°C in LB and BHI media, respectively. The selected isolated colonies were suspended in sodium chloride (NaCl 0.9%) and the turbidity of a growing bacterial suspension was estimated with the McFarland standard, which was equal to a value of 0.5. In the culture tubes containing medium (growth control), AgNPs were dispensed at 1X or 2X of the MIC, and tubes without AgNPs served as a control. Culture tubes inoculated with the bacterial suspension were incubated until a cell count of ∼106 CFU/ml was attained. In order to achieve this, the cultures were kept at 37°C for 24 h [19]. Aliquots were removed for serial dilutions at the indicated time and the number of CFUs/ml was determined on LB/BHI agar. The absorbance at 600 nm wavelength (O.D600) was determined after serial dilution with PBS [20].
Biofilm Inhibition Assay
To determine the antibiofilm potential of as-fabricated AgNPs, overnight cultures of E.coli and S. aureus were grown in fresh sterile LB and BHI broth. Sterile broth (500 μl) was dispensed on a glass coverslip positioned inside wells of the 6-well microtitre plate and inoculated with log-phase bacterial culture aliquots (106 CFU/ml). The biofilm was allowed to grow for 24 hours at 37°C. To eliminate non-adherent cells from a mature biofilm, sterile PBS was employed to wash the film. The AgNPs suspension (corresponding to MIC value) was spread onto the biofilm and incubated for 3 h at 37°C without agitation. The same concentration of AgNO3 solution was used as a control. For staining the bacterial biofilm grown on the coverslip, it was first treated with 1% paraformaldehyde (PFA) followed by exposure to the fluorescein isothiocyanate dye (FITC). The biofilm was washed gently using sterile phosphate-buffered saline (PBS). The anti-biofilm activity of as-synthesized AgNPs was examined based on the determination of residual fluorescence acquired by the bacterial biofilm using a Zeiss Axiocam fluorescence microscope (40x magnification) [21].
AgNPs Cytotoxicity: Haemolytic Effect on Erythrocytes (RBCs) for Assessing Intrinsic Cytotoxicity of As-formed AgNPs
The hemolytic activity of as-fabricated AgNPs against RBCs was determined to assess their cytotoxic effects. We followed a standard protocol, described elsewhere, to determine the hemolytic potential of as-formed AgNPs [22,23]. A known volume of blood (3 ml) was taken from a healthy person and collected in ethylene-diamine tetra-acetic acid (EDTA) containing PBS. The sample was centrifuged for 10 minutes at 1500×g. The supernatant, along with the buffy coat, was removed and the pellet (containing the RBCs) was washed with PBS by centrifugation at 1500×g for 5 minutes. A 50% hematocrit was prepared by diluting the RBCs pellet (packed cells) with an equal volume of PBS. The incurred RBC lysis was measured by incubating the RBC suspension for 1 hr at 37°C with the concentrations of AgNPs varying from 0.5 to 250 µg/ml. The incubated samples were then subjected to centrifugation at 1500g, and the obtained supernatant was assessed and analyzed for the released hemoglobin using a UV spectrophotometer (λmax = 576 nm).
The following equation was used to calculate the percent hemolysis:
% Haemolysis={(Abs (t) – Abs(c))/ (Abs (100%)-Abs(c))} ×100
Where Abs (t) is the absorbance of supernatants obtained from samples mixed with AgNPs, Abs(c) is the absorbance of supernatant from control (PBS), and Abs (100%) is the absorbance of supernatant of the control mixed with (1% Triton X-100), which causes the total lysis of RBCs [24].
Cell Viability (MTT) Assay for Assessing Cytotoxicity of AgNPs
The MTT test is employed to determine the cell viability, proliferation, and cytotoxicity of the living cells. The MTT assay estimates the mitochondrial functioning of the healthy live cells, where the enzyme present in the mitochondrion helps in the conversion of MTT (3-(4, 5-dimethylthiazol-2-yl) – 2, 5-diphenyl tetrazolium bromide) into the formazan crystals. Following a standard protocol, PBMCs were isolated and seeded in a 96-well plate for an overnight period. An increasing amount (concentration) of AgNPs were dispensed into PBMCs harbouring wells. The treated PBMCs were incubated in a CO2 incubator at a temperature of 37°C with 5% of CO2 and 90% relative humidity for 24 hours. After 24 h, 10 μl of MTT solution (5 mg/ml) was put in each well and further incubated for another 4 h. The formazan crystals formed were solubilized in 200 µl DMSO. A Genetix580 microplate reader was used to measure absorbance at 570 nm after 10 minutes (USA). The untreated sets of PBMCs served as a control. To validate the data, the MTT experiment was repeated at least three times. The following formula was used to convert OD values of culture into percentage viability.
Cell viability (%)=[ODSample /ODControl] ×100
Results
Characterization of As-fabricated AgNPs
UV-VIS Spectroscopic Study
The reduction of silver ions (Ag+) into silver particles (Ag0) was observed after the addition of AgNO3 to freshly prepared Pulicaria jaubertii leaf extract at room temperature. The green synthesis of the AgNPs can be followed by determining the colour change (from the original yellow to light brownish to dark brown)as a function of the surface plasmon resonance (SPR) phenomenon. The AgNPs were scanned in the range of 300–700 nm using UV-VIS spectroscopy. Due to the concordant oscillation of the core metal electrons (associated with nanoparticles) in resonance with the light wave; the associated free electrons participate in the SPR with the incident electromagnetic radiation. The λmax of as-fabricated AgNPs was observed to be approximately 465 nm (Figure 1).
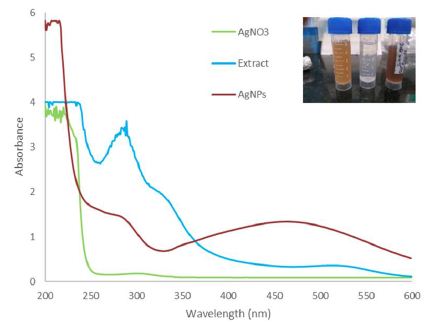
Figure 1: UV-VIS spectrum showing the characteristic features of the as-synthesized AgNPs fabricated by Pulicaria jaubertii leaf extract (λmax ~465 nm).
FTIR Spectroscopic Characterization of As-synthesized AgNPs
The FTIR study was executed to identify metabolites that might be participating in the reduction of Ag+ ions. FTIR spectroscopic study specified the presence of various functional groups in the extract that worked as reducing and capping/stabilizing agents during nanoparticle production (Ag+ to Ag0). The extract exhibited bands at 3200, 2855, 1602, 1514, 1438 and 597 cm−1, whereas the AgNPs exhibited bands at 3399, 2367, 1596, 1244, 1120, and 597 cm−1 (Figure 2). Slight differences were observed between the relative intensities of the neat extract and the AgNPs, where the spectrum shoulder was found to be slightly shifted. The peak at 3399 cm−1 in the FTIR spectrum of AgNPs was ascribed to the stretching vibration of the O-H stretch, a functional group in alcohols/phenols. The absorption band at 2367 cm−1 was ascribed to a thiol as a medium single-bonded S-H stretching. The appearance of an absorption peak at 1596 cm−1, which might be an N-H bend attached to primary amines. The spectral peak at 1244 cm−1 was assigned to a single-bonded C-H wag. The absorption peak at 1120 cm−1 was specific for the vibration of the C-N stretch attributed to aliphatic amines. The absorption band at 597 cm−1 was assigned to a medium single-bonded C-Br stretch attributed to alkyl halides.
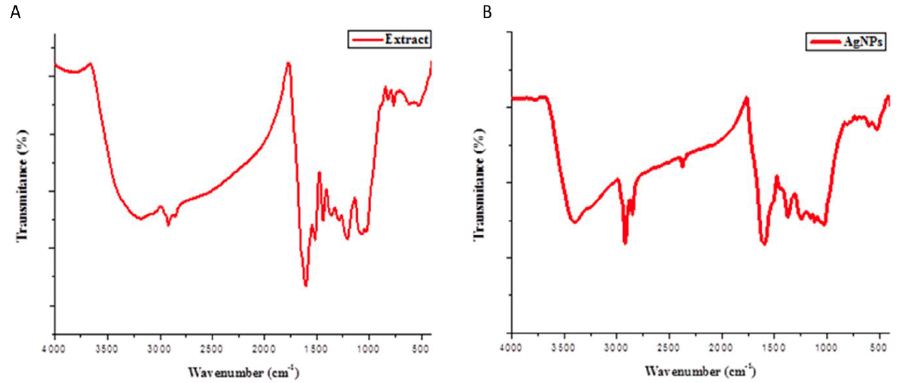
Figure 2: FTIR spectrum of as-fabricated AgNPs showing the characteristic features of associated functional groups accountable for the reduction and capping/stabilization of AgNPs. A) FTIR spectrum of Pulicaria jaubertii leaf extract. B) FTIR spectrum of as-fabricated AgNPs synthesized by incubation of plant leaves extract with precursor AgNO3 solution.
DLS and Surface Charge Analysis of As-synthesized AgNPs
The size of AgNPs as determined by DLS was found to be in the range of 40 to 90 nm (Figure 3A). The zeta potential (AgNPs surface charge) value of as-fabricated AgNPs was around -21 mV as shown in Figure 3B. The stability of the as-synthesized AgNPs has a great deal of correlation with the zeta potential value lying between +30 mV and -30 mV. The nanoparticles with a specific surface charge experience a repulsive force, which in turn prevents agglomeration of the nanoparticles.
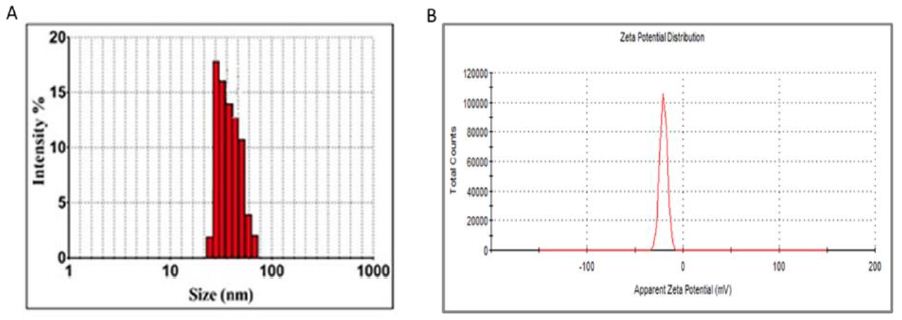
Figure 3: DLS and zeta potential of as-fabricated AgNPs. A) The particle size distribution of as-fabricated AgNPs was assessed by DLS analysis depicting particle size of the as-synthesized AgNPs ranging from 40 to 90 nm. B) The zeta potential of as-fabricated AgNPs was found to be around -21 mV which is representative of the surface charge of AgNPs.
TEM and SEM Electron Microscopic Studies
The size and shape of as-fabricated AgNPs were analyzed through TEM and SEM (Figure 4A and 4B). SEM analysis of as-fabricated AgNPs revealed a spherical shape, while some populations had ovoid or elliptical shapes (Figure 4A). The TEM micrograph showed that the as-fabricated AgNPs had size dimensions between 20 and 50 nm (Figure 4B). The particles were arranged in a clustered pattern. Based on EDX analysis, the elemental composition and relative abundance of the synthesized AgNPs were determined (Figure 4C and 4D). The total chemical composition and purity of as-synthesized AgNPs were assessed by the EDX spectrum. The percentage of the silver metal as compared to other chemical elements was found to be significant. The reduced AgNPs were analysed using EDX, which showed an optical absorption-specific peak at 20 keV.
The EDX analysis displayed the relative percentage composition of many components, including silver (Ag) 50.73%, oxygen (O) 39.58%, gold (Au) 7.75%, and silicon (Si) 1.94%. The residual elements act as capping agents on the surface of AgNPs (Figure 4 and Table 1).
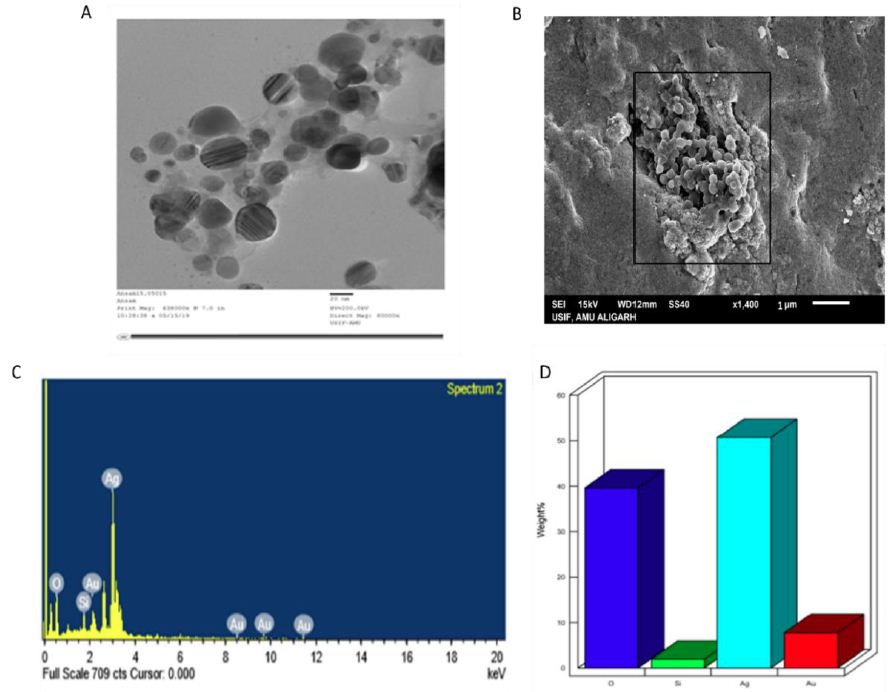
Figure 4: TEM and SEM analysis of as-synthesized AgNPs. A) Representative TEM image of as-fabricated AgNPs fabricated by employing Pulicaria jaubertii leaf extract. TEM image showed that the as-fabricated AgNPs had predominantly spherical, ovoid, or elliptical shapes with particle sizes of 20-50 nm. B) SEM analysis of the as-fabricated AgNPs. C) Determination of elemental compositions of as-fabricated AgNPs. The percentages of Ag, O, Au, and Si in the as-fabricated AgNPs were found to be 50.73%, 39.58%, 7.75%, and 1.94% respectively by employing EDX (equipped with SEM-EDX). D) The elemental composition of the as-fabricated AgNP, is depicted in the form of a bar graph.
Table 1: Characterization of as-fabricated AgNPs fabricated by employing Pulicaria jaubertii leaf extract
|
Sample |
UV-VIS Spectroscopy λmax |
DLS (Size range) |
Zeta-potential (mV) |
PDI |
TEM (Size-range) |
| AgNPs |
465 nm |
40-90 nm |
-21 |
0.4 |
20-50 nm |
Antibacterial Activity of As-fabricated AgNPs
MIC value of As-fabricated AgNPs
In order to assess their antimicrobial efficacy, we estimated the MIC of the as-fabricated AgNPs against gram-negative and gram-positive bacterial pathogens. The MIC value was found to be 12.5 μg/ml for E. coli and 25 μg/ml for S. aureus. The as-synthesized AgNPs showed significant antibacterial potential at par with positive drug control (Gentamycin). Table 2 shows the MIC of as-fabricated AgNPs against the two test bacterial microbes.
Table 2: MIC value of AgNPs against bacterial strains
|
Bacteria |
MIC |
|
E. coli |
12.5 μg/ml |
|
S. aureus |
25 μg/ml |
Antibacterial Potential of As-synthesized AgNPs as Determined by Agar Well Diffusion Method
The antibacterial efficacy of as-synthesized AgNPs against E. coli and S. aureus was also assessed by the agar well diffusion method. We determined the antibacterial activity of AgNPs at increasing concentrations (12.5, 25, 50, and 75 μg/ml). The drug gentamycin (at a concentration of 10 μg/ml) was used as a standard control antibiotic (Figure 5A and B). The antibacterial activity of as-fabricated AgNPs was deciphered from the zone of inhibition (mm) (Table 3). It was found that the antibacterial activity of as-fabricated AgNPs increased significantly in a dose-dependent manner. The relatively large zone of inhibition in E. coli in comparison to S. aureus suggests that AgNPs are more effective against the former. The co-exposure of the bacteria, with a combination of as-synthesized SNPs and standard antibiotic (gentamicin), was executed to establish the synergistic effect of the combination against both E. coli and S. aureus. The AgNPs combination with gentamicin showed a significant increase in the inhibition zone in agar plates.
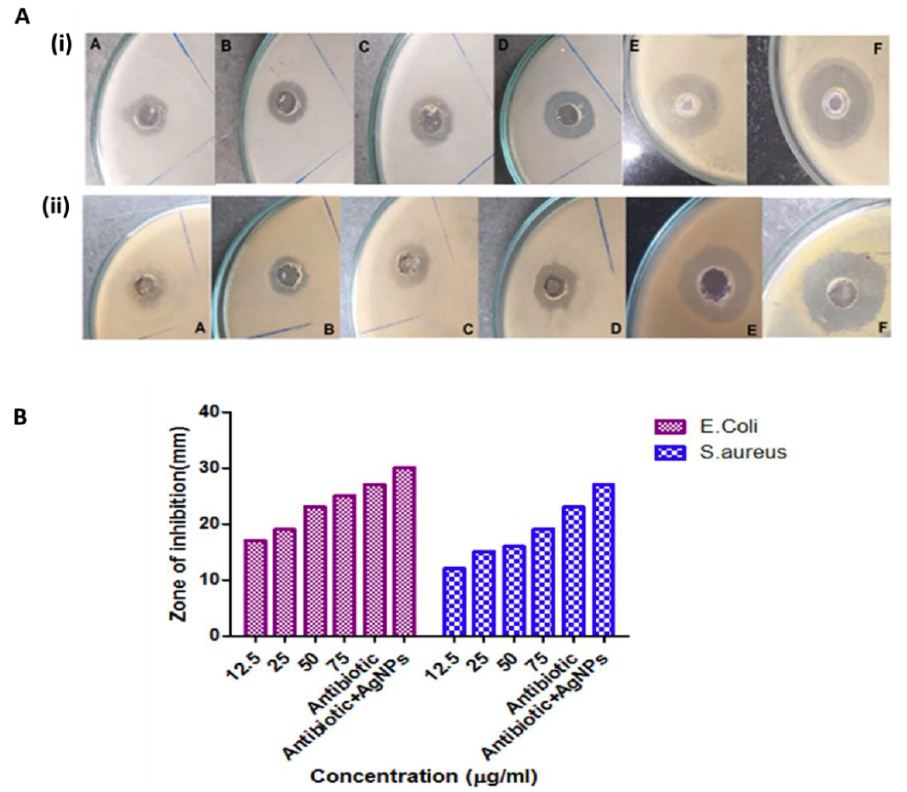
Figure 5: Antibacterial activity of increasing concentration of as-synthesized AgNPs at various concentrations, such as (A) 12.5 µg/ml, (B) 25 µg/ml, (C) 50 µg/ml, (D) 75µg/ml, (E) antibiotic (10µg/ml), and (F) combination of AgNPs (75 µg/ml) + gentamicin (5 µg/ml), against both bacteria i.e., E.coli and S. aureus was checked by using the agar well diffusion method.(A) Zone of inhibition (in mm) was measured for increasing concentrations of as-fabricated AgNPs against the bacterial strain E. coli (i) and E. Aureus (ii). (B) Representative bar graph showing zone of inhibition (mm) corresponding to increasing concentrations of AgNPs against E. coli and S. aureus. The combination therapy (AgNPs along with antibiotics) led to synergistic effects of the mixture by several folds as compared to the precursor formulation.
Table 3: The antibacterial effect of as-fabricated AgNPs was deciphered from the zone of inhibition (in mm) by employing the agar well diffusion method
|
AgNPs Concentration (μg/ml) |
Zone of inhibition (mm) |
|
|
E. coli |
S. aureus |
|
|
12.5 |
17 |
12 |
|
25 |
19 |
15 |
|
50 |
23 |
16 |
|
75 |
25 |
19 |
|
10 (Gentamicin) |
27 |
23 |
|
AgNPs (75) + Gentamicin (5) |
30 |
27 |
Analysis of Time-kill Kinetics of As-synthesized AgNPs
The rate of bacterial killing by the as-fabricated AgNPs was assessed by the time-kill curves. The AgNP formulations were used at 1×MIC and 2×MIC in both the bacteria i.e., E. coli and S. aureus. After 12 hours of incubation, the AgNPs induced a 99% loss of bacterial viability (Figure 6A and 6B). In other words, the AgNP treatment showed a decrease in the number of cells. This was assessed by measuring the absorbance (O.D.) at 600 nm compared to the untreated control.
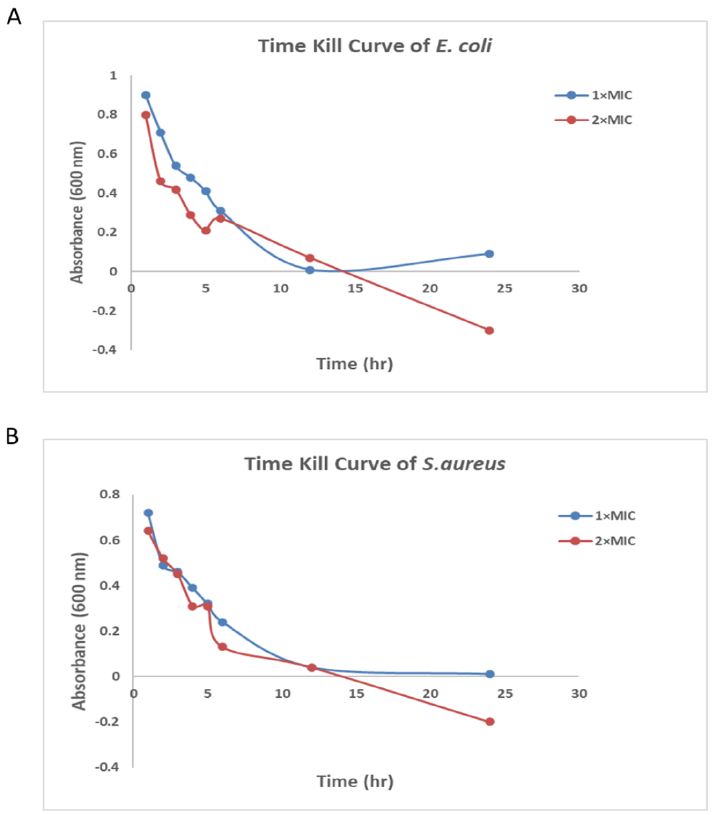
Figure 6: Time-kill curve plot of as-fabricated AgNPs. A) Time-kill curve plot against E. coli in presence of AgNPs 1× or 2× MIC. B) Time-kill curve plot against S. aureus in presence of AgNPs 1× or 2× MIC. The wells without AgNPs served as a control.
Biofilm Inhibition Potential of As-fabricated AgNPs
To evaluate the antibiofilm potential of as-fabricated AgNPs, a biofilm inhibition assay was performed. The fluorescence microscopic images revealed a significant reduction in the formation or maturation of biofilm when bacterial cells were treated with AgNPs (at MIC value). In contrast, there was profuse biofilm formation in the case of control (without treatment of AgNPs), suggesting unhindered proliferation and maturation of the bacterial biofilm (Figure 7).
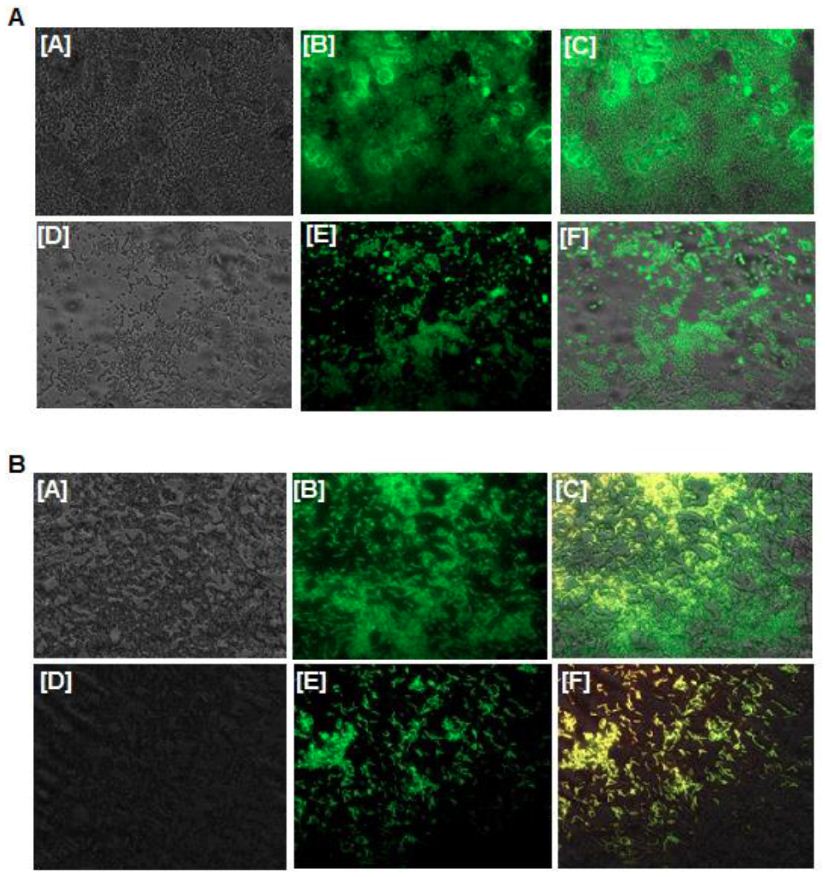
Figure 7: Antibiofilm activity of as-fabricated AgNPs (at MIC value). (A) E.coli biofilm inhibition by AgNPs. Panels [A], [B], and [C] are representative (phase-contrast, fluorescence, and merged) images of untreated(control) biofilm of E. coli, while the images corresponding to panels [D], [E], and [F] show AgNPs treated biofilm of E. coli. (B) The anti-biofilm potential of AgNPs against S.aureus. Panels [A], [B], and [C] are the representative images of untreated (control) biofilm of S. aureus, [D], [E], and [F] panels represent the cells upon their treatment with AgNPs.
As-synthesized AgNPs Manifested Minimal Toxicity on Normal Human RBCs
The RBC lysis (hemolysis) studies showed that as-synthesized AgNPs are safe to be utilized as a therapeutic agent. The detrimental effect of as-fabricated Pulicaria jaubertii leaves decoction fabricated AgNPs on human RBCs was tested by performing a hemolysis assay employing the concentration of AgNPs close to MIC. The AgNPs mediated hemolysis was less than 12%. The toxicity appears to be quite low when compared to the dose equivalent to the MIC value in the instance of E. coli (12.5 µg /ml). However, the hemolysis was ~39% at a maximum concentration (256 µg/ml) of the as-synthesized AgNPs. These findings unequivocally demonstrate that as-synthesized nanoparticles are safe for direct use at low doses (Figure 8).
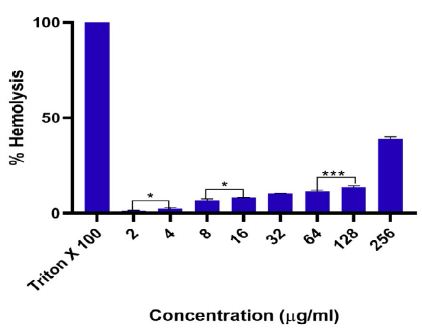
Figure 8: Hemolytic activity of as-fabricated AgNPs. The RBCs were incubated with an increasing concentration of as-synthesized AgNPs. PBS only and Triton X-100 (1%) treated RBCs were referred to as negative and positive control, respectively. The level of damage induced by the AgNPs formulation to human RBCs was evaluated based on the percentage of total erythrocytes that were lysed in each sample. The results are described as mean ± SD; ***P-value ≤ 0.001 and *P-value ≤ 0.05; experiments were performed in triplicate.
Cytotoxicity Assays on PBMC
The as-fabricated AgNPs exhibited minimum cytotoxicity to human peripheral blood mononuclear cells (PBMCs). The results of the MTT assay affirmed that AgNPs did not impose cytotoxic effects on human PBMCs. The AgNPs exhibited a non-toxic nature towards mammalian cells exhibiting a cell survival rate of more than 90% at 32 μg/ml concentration of as-synthesized AgNPs (Figure 9). Even at the maximum concentration of 512 μg/ml, the viability of mammalian cells was maintained at ~45%. However, this amount of AgNPs is not likely to be achieved at a therapeutic level, yet it has little effect on cell viability. The toxicity data establishes the safety aspects of the as-produced AgNPs.
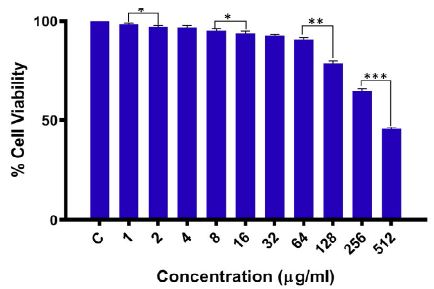
Figure 9: Cytotoxicity assay of as-fabricated AgNPs. Thiazolyl blue tetrazolium bromide (MTT) assay was used to measure the dose-dependent effect of AgNPs on PBMCs.The data are shown as mean ± SD; ***p-value ≤ 0.001; **p-value ≤ 0.01; and; *p-values ≤ 0.05 where, the experiments were performed in triplicate.
Discussion
In the present study, we have presented an environmentally green synthesis method to fabricate silver nanoparticles by making use of Pulicaria jaubertii leaf extract. Pulicaria jaubertii is a medicinally active plant. The data of the present study establish the potential of Pulicaria jaubertii leaf extract to mediate the green synthesis of AgNPs by reduction of core Ag+ salt. Metal salts are prone to undergo reduction by the components of plant extract and eventually get transformed into nanosized particles of varying sizes and shapes. Pulicaria jaubertii leaf extract was found to transform silver salt into nanosized AgNPs. The leaf extract also helped AgNPs formation and its subsequent capping (to avoid aggregation). The nanocrystal nuclei collide with each other to form relatively large-sized nanoparticles [25]. The synthesis of the nanoparticles was found to be accompanied by a change in the color of the suspension [26]. The UV-VIS absorption spectrophotometric study of as-prepared AgNPs revealed a λmax at 465 nm.
FTIR analysis revealed the existence of many functional groups in both Pulicaria jaubertii leaf extract and their subsequent adsorption onto the surface of as-fabricated AgNPs, including carboxylates, hydroxyl, amines, and phenolic functional groups. The occurrence of the functional groups on the surface of AgNPs indicates that they were derived from leaf extract. This also in turn suggests that the above-specified chemicals are not only playing a potential role in particle fabrication, however, also acted as a capping agent to stabilize as-synthesized particles.
TEM analysis revealed that the nanoparticle’s dimension ranges from 20 to 50 nm. Additionally, DLS measurements showed that the nanoparticle size ranges from 40 to 90 nm. In the DLS method, the particle’s average hydrodynamic diameter is determined by using the Stokes-Einstein equation. This is mostly considered a possible explanation for the inconsistency between DLS and TEM-based size determinations. In the former, the size corresponds to solvent-free form, whereas DLS measurements involve hydrodynamic and electro-kinetic parameters [27].
Next, as-fabricated AgNPs were assessed for antimicrobial potency towards gram-positive and gram-negative bacterial pathogens. After 12 hours of incubation with AgNPs, the AgNP formulations induced a 99% reduction of bacterial viability when tested at 1× MIC or 2× MIC. The inhibition of microbes was further confirmed by an agar disc diffusion assay, which revealed a distinct inhibition zone in the agar plate. Gentamicin was used as a standard antibiotic control. The efficacy of as-fabricated AgNPs alone and in combination with gentamycin was also evaluated. When standard antibiotic was supplemented with AgNPs, the antimicrobial potential of antibiotic against microbes increased significantly. The zones of inhibition increased significantly with increasing concentrations of AgNPs.
The biofilm inhibition assay showed enhanced antibiofilm activity of as-fabricated AgNPs against both bacterial strains. The AgNPs demonstrated higher antibacterial efficacy against pathogenic microorganisms E coli and S. aureus. The mechanism behind the antibacterial activity is most likely because of the binding of AgNPs with the bacterial cell wall and causing the generation of free radicals. The nanoparticles disrupt cells by reacting with phosphorus- and sulfur-containing compounds like DNA and proteins [28]. The release of silver ions from the nanoparticles is another potential explanation for the bactericidal effects of silver; that is how the AgNPs degrade the bacterial cell.
Next, a toxicity assay of AgNPs on RBCs and PBMCs was also performed. Despite the very high dose of as-fabricated AgNPs (256 μg/ml), there was less than ~39% lysisof RBCs (Figure 8). Similarly, after being exposed to 32 μg/ml, PBMCs demonstrated cell viability of about 90%. The toxicity study supports the safety aspects of as-synthesized AgNPs.
Conclusion
The proposed bio-mediated synthesis of AgNPs has the adeptness to serve as a potential method for the effective, inexpensive, and non-toxic large-scale manufacturing of AgNPs. Because of their benign and stable nature, and also their antibacterial properties, the as-fabricated AgNPs may be successfully employed in industrial and therapeutic use and could pave the way as an alternative medicine for several disease therapies. However, more studies are needed to investigate its mechanism of action and also to assess its toxicity in in-vivo animal models as well.
Acknowledgement
We are thankful to the co-ordinator, IBU, AMU, Aligarh, for allowing us to avail the department instrument facilities. We are also thankful to Prof. M. Shahis for his help and supply of the microbial strains.
References
- Lee, Sang Hun, Bong-Hyun Jun (2019) Silver nanoparticles: synthesis and application for nanomedicine. International journal of molecular sciences 20: 865. [crossref].
- Mittal, Amit Kumar, Yusuf Chisti, Uttam Chand Banerjee (2013) Synthesis of metallic nanoparticles using plant extracts. Biotechnology advances 31: 346-356.
- Tran, QuangHuy, Anh-Tuan Le (2013) Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives. Advances in Natural Sciences: Nanoscience and Nanotechnology 3: 033001.
- Ojo, OluwafemiAdeleke, et al. (2021) Nanoparticles and their biomedical applications. Biointerface Res Appl Chem 11: 8431-8445.
- Bhattacharya, Tanima, et al. (2022) Novel Green Approaches for the Preparation of Gold Nanoparticles and Their Promising Potential in Oncology. Processes 10: 426.
- Prabhu, Sukumaran, Eldho K. Poulose (2012) Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, toxicity effects. International nano letters 2: 32.
- Moustafa, Hesham, et al. (2019) Eco-friendly polymer composites for green packaging: Future vision and challenges. Composites Part B: Engineering 172: 16-25.
- Gahlawat, Geeta, Anirban Roy Choudhury (2019) A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC advances 9: 12944-12967.
- Albukhari, Soha M, et al. (2019) Catalytic reduction of nitrophenols and dyes using silver nanoparticles@ cellulose polymer paper for the resolution of waste water treatment challenges. Colloids and Surfaces A: Physicochemical and Engineering Aspects 577: 548-561.
- Ragab, Menna M, Ahmed G. Hassabo (2021) Various uses of natural plants extracts for functionalization textile based materials. Journal of Textiles, Coloration and Polymer Science.
- Dwivedi, AmarendraDhar, Krishna Gopal (2010) Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids and Surfaces A: Physicochemical and Engineering Aspects 369: 27-33.
- Dubaie, A. S, A. A. El-Khulaidi (2005) Medicinal and aromatic plants in Yemen, deployment-components of effective-uses. EbadiCenter for studies and Publishing Sana’a-Yemen 127.
- Dubaie, A. S, A. A. Al‐Khulaidi (1993) Studies on the flora of Yemen on the flora of Tihama plain with one figure. FeddesRepertorium 104: 259-265.
- Zhang, Yu, et al. (2021) Green synthesis of NiO nanoparticles using calendula officinalis extract: chemical charactrization, antioxidant, cytotoxicity, anti-esophageal carcinoma properties. Arabian Journal of Chemistry 14: 103105.
- Ashraf, Jalaluddin M, et al. (2016) Green synthesis of silver nanoparticles and characterization of their inhibitory effects on AGEs formation using biophysical techniques. Scientific reports 6: 1-10. [crossref]
- Kelly, Fern M, James H. Johnston (2011) Colored and functional silver nanoparticle− wool fiber composites. ACS applied materials & interfaces 3: 1083-1092. [crossref]
- Roy, Swarup, Tapan Kumar Das (2015) Plant mediated green synthesis of silver nanoparticles-A. A Review Int J Plant Biol Res 3: 1044-1055.
- Saravanan, M, Anil Kumar Vemu, Sisir Kumar Barik (2011) Rapid biosynthesis of silver nanoparticles from Bacillus megaterium (NCIM 2326) and their antibacterial activity on multi drug resistant clinical pathogens. Colloids and Surfaces B: Biointerfaces 88: 325-331. [crossref]
- Zhao, Tong, Michael P. Doyle, Ping Zhao (2004) Control of Listeria monocytogenes in a biofilm by competitive-exclusion microorganisms. Applied and Environmental Microbiology 70: 3996-4003. [crossref]
- Kumar, TKM Prashantha, et al. (2015) Highly efficient performance of activated carbon impregnated with Ag, ZnO and Ag/ZnO nanoparticles as antimicrobial materials. RSC Advances 5: 108034-108043.
- She, Pengfei, et al. (2015) The effects of d-Tyrosine combined with amikacin on the biofilms of Pseudomonas aeruginosa. Microbial pathogenesis 86: 38-44. [crossref]
- Oves, Mohammad, et al. (2013) Antibacterial and cytotoxic efficacy of extracellular silver nanoparticles biofabricated from chromium reducing novel OS4 strain of Stenotrophomonasmaltophilia. PloS one 3: e59140.
- Rauf, MohdAhmar, et al. (2017) Biomimetically synthesized ZnO nanoparticles attain potent antibacterial activity against less susceptible S. aureus skin infection in experimental animals. RSC Advances 58: 36361-36373.
- Vijayan, Smitha, et al. (2016) Antibacterial and Cytotoxicity Studies of Silver Nanoparticles Synthesized by EndophyticFusariumsolani Isolated from Withaniasomnifera (L. ) Journal of Water and Environmental Nanotechnology 1: 91-103.
- [25] Arya, Geeta, et al. (2018) Green synthesis of silver nanoparticles using Prosopisjuliflora bark extract: reaction optimization, antimicrobial and catalytic activities. Artificial cells, nanomedicine, and biotechnology 46: 985-993.
- Kumar, R, et al. (2017) Rapid green synthesis of silver nanoparticles (AgNPs) using (Prunuspersica) plants extract: exploring its antimicrobial and catalytic activities. J NanomedNanotechnol 8: 2.
- Premasudha, Paramasivam, et al. (2015) Biological synthesis and characterization of silver nanoparticles using Ecliptaalba leaf extract and evaluation of its cytotoxic and antimicrobial potential. Bulletin of Materials Science 38: 965-973.
- Hamouda, Ragaa A, et al. (2019) Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatorialimnetica. Scientific Reports 9: 1-17. [crossref]