DOI: 10.31038/CST.2017247
Abstract
Nothing makes sense except considering evolution, by which understanding how life originates it is important to be able to decipher the mysteries that lead to the abnormal behavior of the cancer cell. So far, the emergence of life is explained in religious terms, but in scientific terms, it was not possible. The various existing theories had gaps that could not be resolved. The discovery of unsuspected capacity of melanin transform light into chemical energy, such as chlorophyll in plants; It is a watershed in biology, it turns out that melanin is the missing link in the chain of events that leads to the emergence of life. Now the Outlook is different, we can advance faster in the study and treatment of diseases that currently constitute a scourge to mankind, such as cancer. We are on the threshold of a new era in biology and in medicine.
Introduction
The unsuspected bio-energetic role of melanin has been described previously [1]. Electromagnetic radiation, melanin and water are substances that do not present any data that allow us to think that they have some thought as an evolution process. You could say that its main physical and chemical characteristics are the same since the beginning of time.
What we call evolution, can realize to the biochemical scaffolding that nature has patiently developed over eons of years, to increasingly optimize the properties of light, melanin and water, which have not changed a whit.
Eukaryote cell is becoming more sophisticated, biochemical gear is increasingly complex, and what is most striking, the power supply remains the same since the beginning of time. This is: the first living entities, had similar amount of available chemical energy derived from the melanin that human beings today.
It is something that has not changed despite the passage of time. What, has changed continuously over time, is the number of molecules, organelles, enzymes, etc., have been bolted together to make increasingly more sophisticated, increasing the complexity of the cell. And the term sophisticated, applied to any system; means that it contains many parts.
Continues to call me the attention that the power source is still, on the one hand, the sunlight mainly, on the other hand, melanin, which has not changed in the slightest despite the course of millions of years; and so on the perfect substrate which is water.
It is interesting that one of the elements, melanin; is completely dark and we can’t see through it, either with the naked eye or with instruments, and to this we attribute the fact that its formula is not known. But, on the other hand; water is completely transparent, we observe it with the naked eye, and yet we don’t understand as the hydrogen and oxygen atoms that compose it are organized, due to diverse factors, i.e. because its arrangement is dynamic and not uniform.
The nature of light, we neither speak, don’t even understand if it is a wave or a particle, or both. So, from three natural phenomena which we know little or nothing, life originated. Melanin is the missing link between the different theories that try to explain how emerged those we named life.
Light, Melanin, and Water
The physical and chemical properties of light, melanin and water whether we understand them or not, they possess characteristics that seem to be immutable, they inner dynamic had not change in the slightest despite the passage of time. And it seems paradoxical that the union of these quite stable elements generates the very dynamic phenomenon that we call life; which is characterized by constant changes, in all times, everywhere.
Charles Darwin said that life originated in warm water, with low oxygen levels, and without any other form of present life. And it is all spent out there, because the amniotic fluid is warm water with low oxygen levels and without any other way of life.
The evolution of the human eukaryote cell, requiring billions of years of evolution, based on primitive cells, is repeated in each pregnancy, and reducing days. Thus, the initial cells during pregnancy are primitive cells, whose structure is not as complex as mature or specialized eukaryote cell.
But despite differences that may be, at least in appearance; different cell lines retain the same fundamental process of generation and distribution of energy from the melanin.
In eukaryotes normal cells, of any cell line; organelles are very similar, even in size; which reflected a surprisingly uniform pattern, which cannot be a result of chance. And that consistency comes from the origin of life, as both light, melanin, and water, are very dynamic elements but do not change, do not seem to evolve in the least; It seems that they reached perfection and do not need to go further.
What if it evolves, and does so constantly, all the time, is what we call life. And one of the features of the evolution, is the increasingly complex management, in many respects, from the chains of carbon mainly from photosynthesis-derived glucose, this is: a homogenously arrangement of carbon, oxygen, and hydrogen atoms, with CnH2nOn as general formula, and whose most soluble example is glucose. While more advanced is a cell in the evolution, more efficient is the way in which atoms and carbon chains entwine each other forming increasingly intricate and complicated processes and structures, but in the end, reflects an increasingly efficient use of the energy that emanates from the melanin in the form of molecular hydrogen and high-energy electrons. A kind of energy astonishing accurate.
The proliferative cell
It is a deep-rooted dogma that normal differentiated cells, rely primarily on mitochondrial oxidative phosphorylation to generate energy needed to impel cellular processes.
So, it is not surprising that the metabolism of embryonic cells present findings consistent with the Warburg effect [2], or aerobic glycolysis. Which is an inefficient way to generate adenosine 5´-triphosphate (ATP), allowing to incorporate nutrient into biomass, rather than efficient and too much complicated, ATP production.
However, mitochondria and ATP biological functions are regulation of temperature and phosphate levels, but no energy production. Therefore, ATP biological function is regarding temperature control and phosphate levels, compounds that are chemically unstable but thermodynamically stables.
Thereby, embryonic cells require generating biomass so quickly, and gradually more and more complicated processes appear inside these cells, reflected, i.e.; by the increasing mitochondria number, whose function is the temperature control as much as possible, due to the chemical reactions inside normal differentiated cells are astonishing accurate, and temperature is no exception.
By other hand, uncontrolled proliferation is due to the cells that turn back in evolution, being the explanation due to the generation and distribution of melanin´s energy has been affected chronically.
Cancer cells are not generally controlled by normal regulatory mechanisms [3]. Thereby, the characteristic disordered tumor growth is dependent of difficult-to-understand mixture of local factors. A good example are the processes involved in angiogenesis and vascular remodeling implied in the vascularization of malignant tumors.
Total spectrum of morphogenic and molecular events required to form a neovascular network are way beyond our abstraction capacity, because casualness is a significant factor. And worst, those events are significantly different of normal vasculogenesis.
But in the light of the bio-energetic unsuspected role of melanin, we can reconsider such processes initiating the analysis as follows: the blood cannot carry energy, and on the other hand, the amount of chemical energy available in a cancer cell, is significantly lower than in a normal cell, therefore we have a series of events that reflect a step backwards in the evolution of the diseased cell and their chemical and anatomical, physical characteristics indicate that address.
While more evolved it is a cell, its operation is farther from random, and vice versa.
The intricacy of the processes that make up a specialized cell, they make these increasingly more and more precise, and one of the purposes of evolution is gradually reducing the interference of random in intracellular biochemical processes. Since any alteration in its sequence, temporality, or spatial location, would have devastating effects on perfectly than a specialized eukaryote cell represents.
Anti-vascular therapy of cancer
It has been over 30 years since Judah Folkman hypothesized that tumor growth is angiogenesis dependent [4]. But after years of clinical trials based in Dr. Folkman theory, results have been disappointing. Inhibition of VEGF, the main molecular mediator in capillary sprouting has been insufficient to halt tumor progression permanently in many cancer types.
The reasons can be diverse. i.e. the existence of multiple vascularization mechanisms and additional growth factor pathways. In our experience, the main cause of this failed therapeutic focus is that blood cannot carry on energy, just cell´s building blocks as glucose, aminoacids, lipids, etc., and CO2.
Cancer cells
Most solid tumors display distinct aneuploid karyotypes (abnormal chromosomal numbers) and frequently miss-segregate whole chromosomes in a phenomenon called chromosomal instability (CIN). CIN positively correlates with poor patient prognosis, indicating that reduced mitotic fidelity contributes to cancer progression by increasing genetic diversity among tumor cells [5].
Mechanisms leading to the loss of mitotic fidelity in CIN are not known. A common mitotic defect in tumor cells with CIN is the persistence of erroneous attachments of chromosomes to spindle microtubules (merotely).
In normal diploid cells erroneous attachments arise spontaneously and are efficiently corrected to preserve genomic stability. Paradoxically, kinetochore microtubule attachments in cancer cells with CIN are more stable than those in normal diploid cells, thereby, accounting for the persistence of mal-attachments into anaphase, causing chromosome miss-segregation. Seems that cancer cells have a diminished capacity to correct erroneous kinetochore-microtubules attachments; a dysfunction with a widespread occurrence of CIN in tumors.
The mechanisms involved in ploidy protection and genomic integrity are highly complex, not understood, and astonishingly accurate. In example, initiation of a new round of DNA replications should be restricted until after completion of the previous mitosis. Thereby DNA replication depends of biochemical pathways that occur exactly in time, space, location, amount; etc., in an amazing way; but are largely unknown.
The failures in these poor-understood regulatory mechanisms are generalized. Therefore, the plausible explanation is energy. Other theories proposed in the scientific literature, trying to explain abnormalities observed in cancer cells, has been proved elusive, given the inherent complexity of specialized eukaryotic cell after four billion yeas of evolution.
However, we are in front of a new era in the molecular biology of cancer cells; thanks to unsuspected bio-energetic role of melanin. And the explanation seems as quite simple: a specialized eukaryotic cell whose levels of generation and distribution of energy (from melanin) are impaired by contaminated water, polluted air, pesticides, herbicides, fertilizers, transition metals, heavy metals, addictive drugs, solvents of diverse types, industrial waste, stress, etc., tends to turn back in evolution. Simply because the complicated biochemical cell scaffolding that gives vital and highly-ordered support to the poorly understood specialized eukaryotic cell, requires astonishingly accurate amounts of energy.
Insofar as the available quantities of energy inside the cell to reduce enough, for example, in time and form, the very complicated biochemical pathways start to collapse in a disorderly way. Apparently, keeping only the most basic functions; or at least the first that appeared during the evolution, which gives us an idea that cell not is being able to keep relatively recent occurrence mechanisms.
In humans, aneuploidy is linked to pathological defects such as development abnormalities, mental retardation, or cancer, but the underlying mechanisms remain elusive. There are many types of aneuploidy whose origin remains unknown. A common response independent of the type of aneuploidy can be a novel target for cancer therapy.
Genomic stability requires that genetic material must be equally distributed between the two daughter cells during mitosis. An apparently straightforward process, by far beyond our abstraction capacity. By other side, an aberrant number of chromosomes, or aneuploidy, has been recognized as a feature of human malignancies for over a century, but until today without compelling evidence of causality.
Molecular logic underlying proper and aberrant chromosome segregation are extremely complex. It is undoubtedly that chromosome instability is detrimental for the fitness and survival of normal cells, being also the hallmark of cancer cells. Paradoxically, cells with an elevated proliferative potential, are also highly aneuploid.
Theoretically, Aneuploidy is a direct consequence of chromosome segregation errors in mitosis, while structural aberrations are caused by improperly repaired DNA breaks, but also exists a cross-talk between them. The long-lasting efforts to selectively inhibit proliferation of tumor cells has been infructuous.
Toxins against microtubules exert anti-tumor effects in some patients, but it is unclear its action´s mechanism. Apparently, these compounds acts interfering with microtubule dynamics during mitosis activates the spindle checkpoint, causing a prolonged mitotic arrest. Thereby, cells either then undergo death in mitosis or slippage; returning to interphase without dividing; but it is unclear what dictates the balance between these two fates.
Therapeutic inhibition of major mitotic kinases and kinesins has given a modest clinical activity at best.
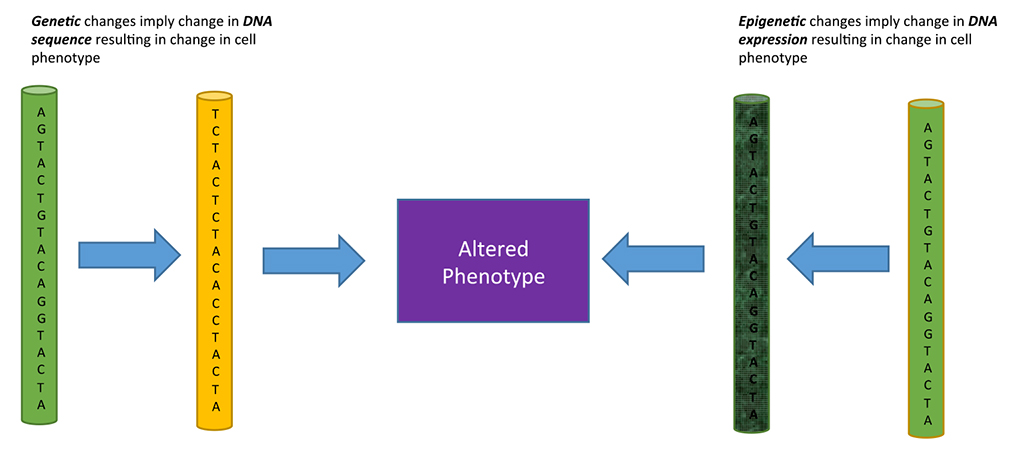
Thereby, the maintenance of chromosomal stability involves the whole cell, not only genes. So, generation and distribution of energy from melanin has a fundamental role in cell biology (Figure 1).
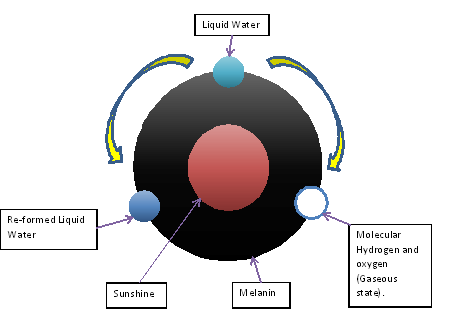
Figure 1. Diagram of the hitherto unknown intrinsic capacity of melanin to transform visible and invisible light into chemical energy, dissociating the water molecule, as chlorophyll in plants.
The energy that melanin provides incessantly, night and day, in a quite consistently form, both in and outside the cell, explains finally, the origin of life.
In chlorophyll, the water dissociation is irreversible; but in melanin is reversible. The process is as follows:
2H2O → 2H2 + O2 →2H2O + 4e–
The liquid water is sublimated by melanin into its gaseous components molecular Hydrogen (H2) and Oxygen (O2) in astonishing and accurate way, this is: always the product of the water dissociation is molecular hydrogen and molecular oxygen. Thereby, it is a quite precise process even in a diversity of environmental conditions, because melanin acts also as attemperator of surrounding sudden changes, for instance, a significant increase or decrease in the amount of light; keeping the output of H2 and O2 between narrow ranges.
The biochemical process of cell (and body) is amazing accurate, otherwise life is not possible; but this astonishing precise start since the very first step of life that is the energy production. Evolution can be interpreted as the increasing and gradual organization of the distribution of energy that is more and more exquisite, efficient; and precise as time pass by. Recall that is the same energy since the beginning of time, both in nature and amount.
The specialized eukaryotic cell does not generate more energy from melanin, because melanin energy generation has a top, a guarded proportion; unless more melanin is added to the system. Instead it is much more efficient in the distribution and thereby using of H2 and e- that the melanin dissociation and re-formation of water produces constant and exactly during night and day.
Eukaryote cell, mature, specialized; It is a whole that works perfectly in every one of its parts, starting from the generation and distribution of energy; which has been patiently optimizing over millions of years of evolution. You could say that nothing or almost nothing of what happens inside a specialized cell is the result of chance.
In any system, not only in biology; energy, defined as everything that produces a change; It is the most sensitive segment as an alteration in this area produces widespread failures, such as that seen in cancer cells.
Hence how difficult that has been to develop a successful therapy in cancer cells, it seems virtually impossible, because when designing strategies that act on a certain part of the cell, for example microtubules; the cell simply changes its behavior, which is now largely influenced by random, totally opposed to a normal cell; and quickly becomes resistant to instituted treatment.
And the explanation is that simply the imbalance now is expressed differently, but the actual background of the problem doesn´t change, and the proof is that proliferation does not diminish. Many of the deviations observed in a cancer cell should be taken care at the same time, so cell tends to function normally, as it has millions of years, millions of times.
Experiments in this regard have shown that partial reparation of the altered intracellular processes is not the solution, it is necessary to address the entire e ideally at the same time.
This is not currently possible, except to increase the generation and distribution of energy that comes from the melanin, and since all the biochemical processes that occur inside the eukaryote cell depend entirely on the H2 and e–, to normalize these levels, all processes tend to operate normally, because ultimately, they are chemical reactions quite exact, that may not happen or inadequately occurs if the power level is not suitable.
QIAPI 1® and Cell Proliferation
The following Tables (1-4) show results of experiments carried out in cultured human cells. The results are part of the development of medication implemented in our laboratory, and which is in phase of patent in several countries, which has already been granted in several of them.
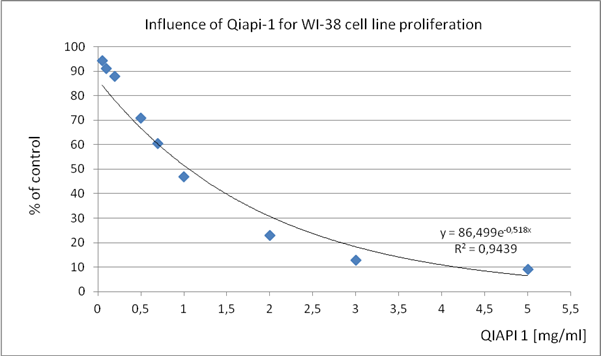
Table 1. WI-38 (human diploid cell line from normal embryonic (3 months gestation) lung tissue)
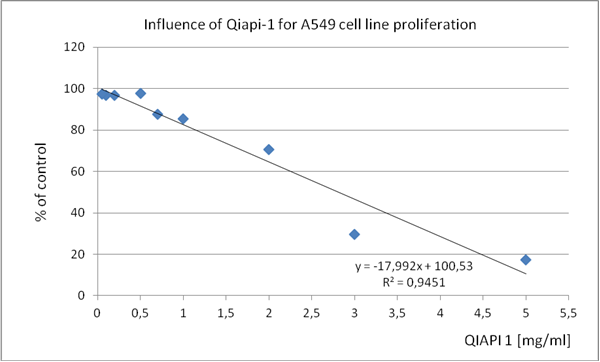
Table 2. A549 (human lung adenocarcinoma epithelial cell line)
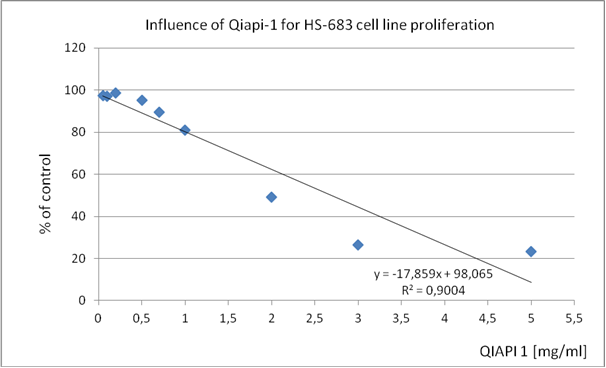
Table 3. HS 683 (human neuronal glioma cell line)
Table 4. Inhibitory concentrations of QIAPI 1®.
| Cell line |
Concentrations of QIAPI 1 inhibit 50% proliferation[mg/ml] |
Concentrations of QIAPI 1 inhibit 99% proliferation[mg/ml] |
| WI-38 |
1,0 |
4,5 |
| A549 |
2,0 |
5,5 |
| HS-683 |
2,0 |
5,5 |
These results present them as a proof of concept, since the mechanism of action of QIAPI 1® is intensifying the dissociation and reformed the water, by the melanin molecule. (Tables 1-4)
The similarity in the results, although cultures of different cell lines, support our theory that acting on the generation and distribution of energy, cells, regardless of human classifications, they tend to recover complex order that is required so that their behavior is normal, proper; as millions of years, has done millions of times.
QIAPI 1® is a new therapeutic agent [6] that gradually will appear in the market, as legal requirements should be fitted.
Conclusion
The apparent chaos that reigns in efforts to decipher the bases of the anomalous behavior of cancer cells, appears to take a totally different direction when we do aside dogma that the main source of cell energy is glucose, then the discovery of unsuspected intrinsic property of melanin make visible and invisible light into chemical energy, such as chlorophyll in plants; through the dissociation of the water molecule, it is a watershed in cell biology.
We have a new view in the study of the biology of cancer, and while we move faster in that sense, the benefit that can give every day to numerous patients affected by the disease will be higher.
Acknowledgement: This work was supported by Human Photosynthesis® Research Center.
References
- Solis-Herrera, A. Arias-Esparza, MC. Solis-Arias, RI. Solis-Arias, PE. Solis Arias, Martha P (2010) The unexpected capacity of melanin to dissociate the water molecule, fills the the gap between the life before and after ATP. Biomed Res 21: 224-227.
- Vander Heiden, Matthew Cantley, Lewis, Thompson, Craig B (2009) Understanding the Warburg effect: The Metabolic Requirements of Cell Proliferation. Science 324: 1029-1033.
- Döme, Balázs Hendrix, Mary JC, Paku, Sändor Tóvári, József, Timár, Jozsef (2013) Biological perspectives. Alternative Vascularization Mechanisms in Cancer. Pathology and Therapeutic implications. Am J Path.
- Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285: 1182-1186. [crossref]
- Compton, Duane (2013) Mechanism of chromosomal instability in human tumor cells. Geisel School of Medicine at Dartmouth, Hanover, USA.
- Solis-Herrera, A Ashraf, Ghulam M, Arias-Esparza, MC Solis-Arias, et al. (2015) Biological Activities of QIAPI 1 as Melanin Precursor and its therapeutic effects in Wistar Rats exposed to Arsenic Poisoning. CNS Agen in Med Chem 2: 99-109.