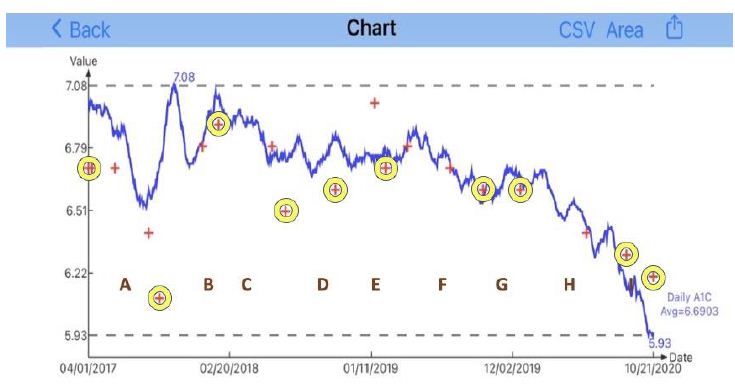
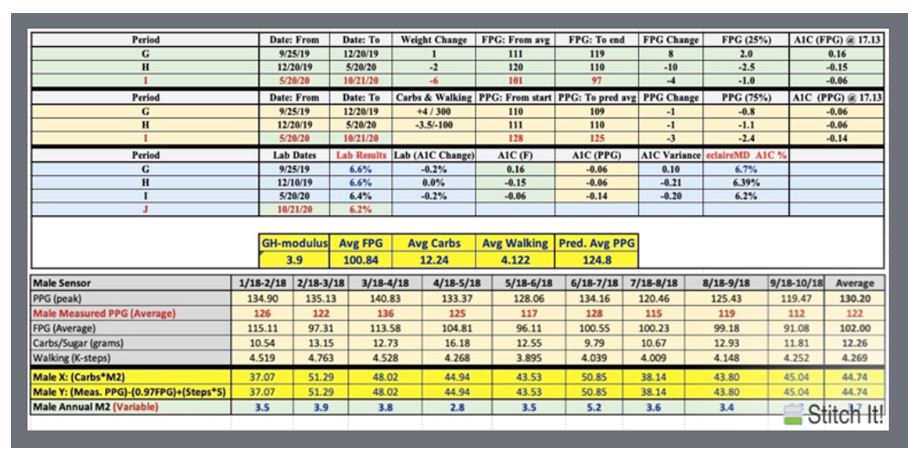
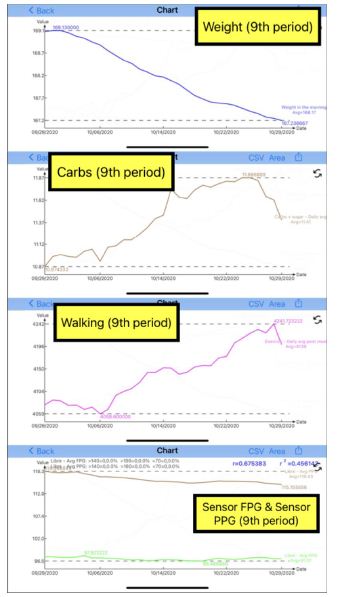
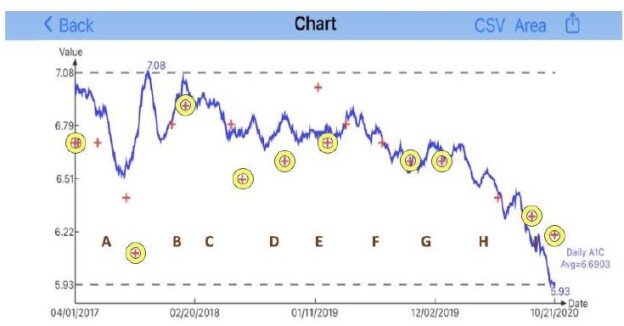
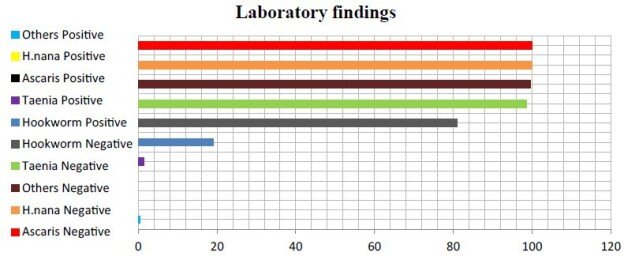
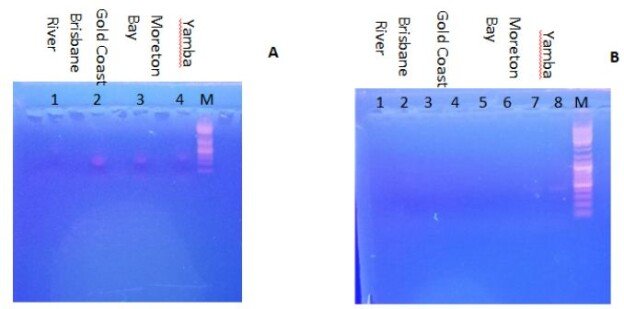
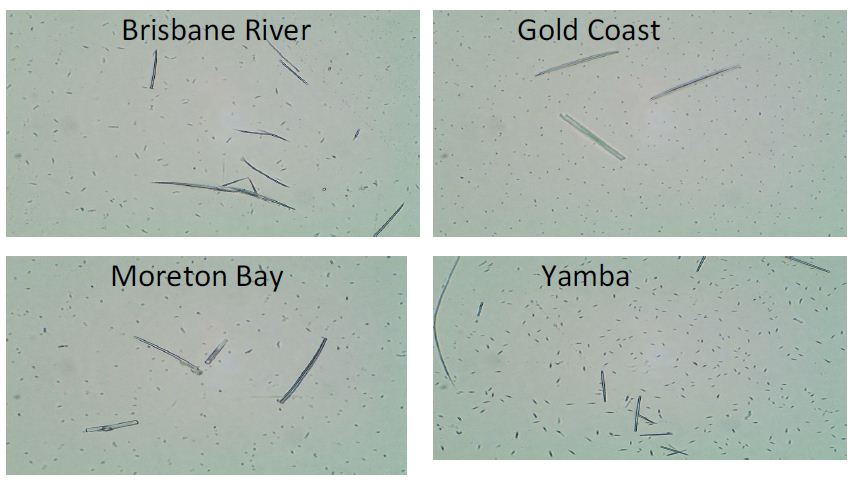
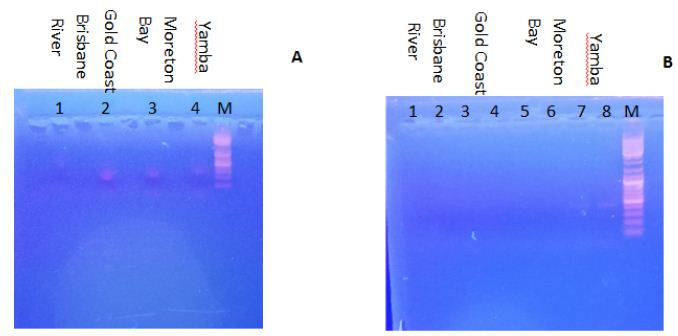
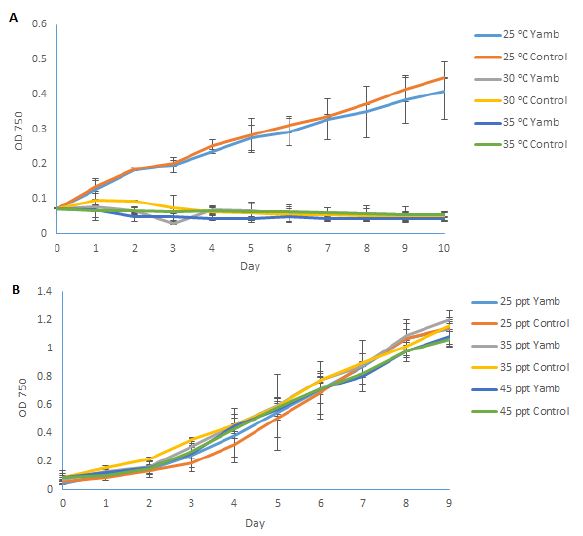
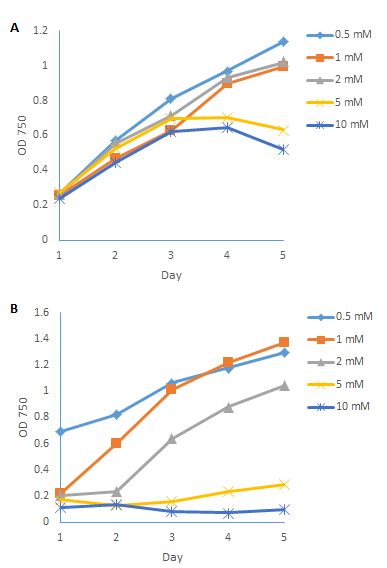
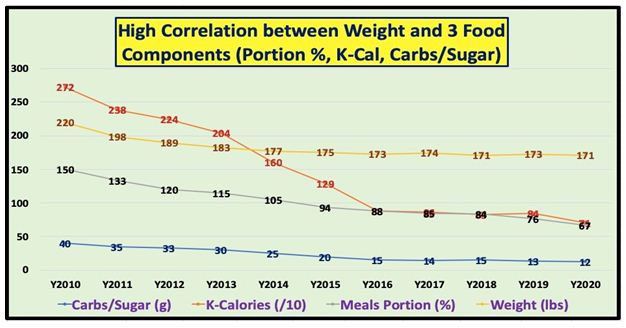
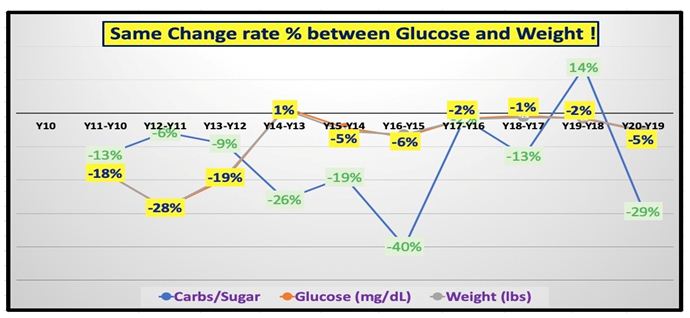
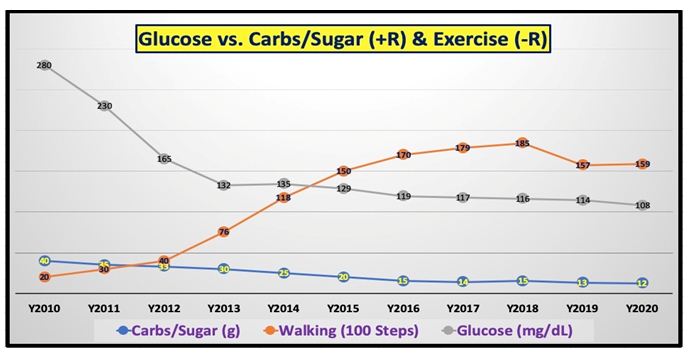
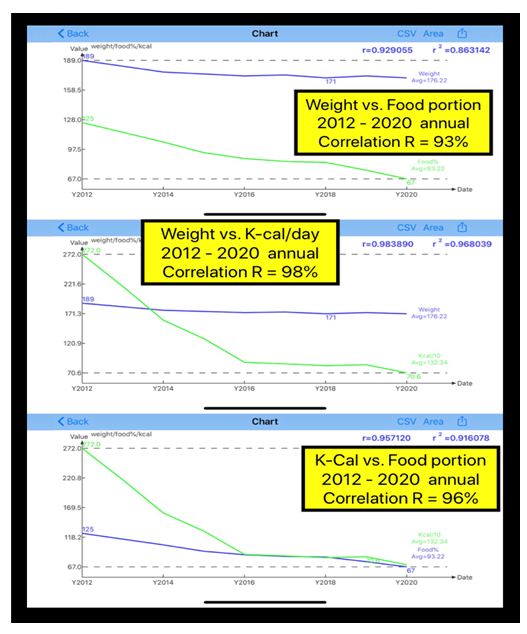
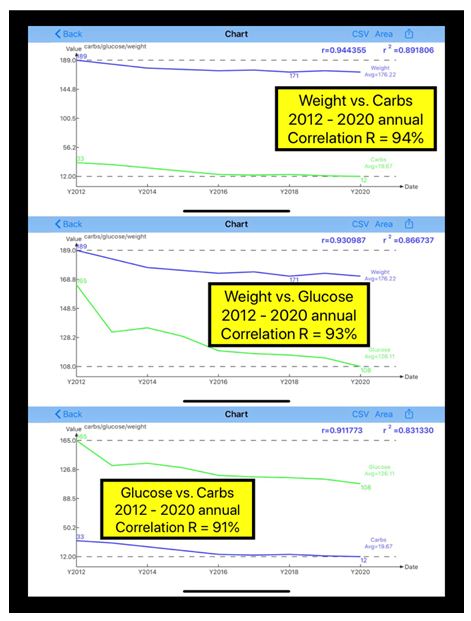
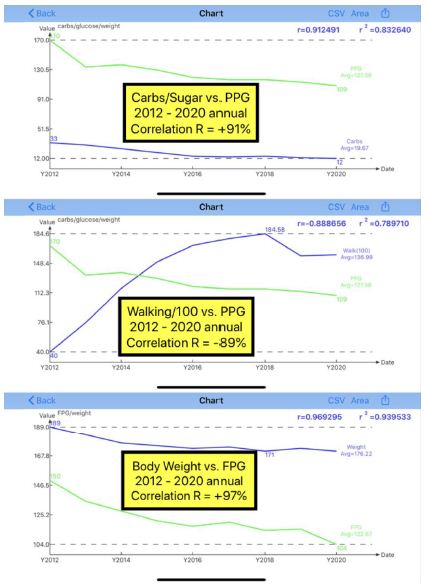
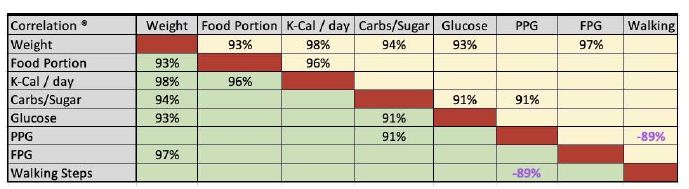
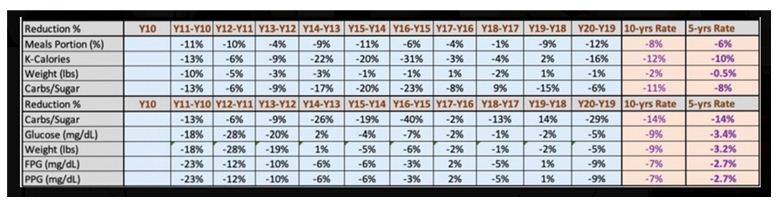
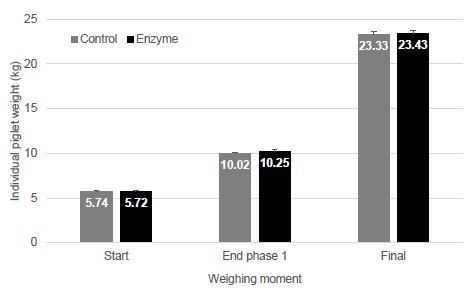
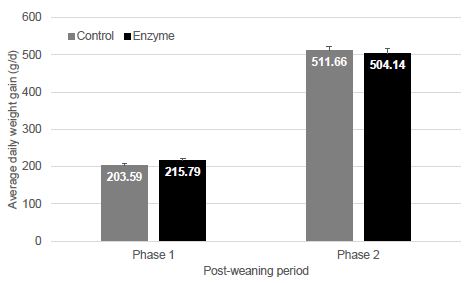
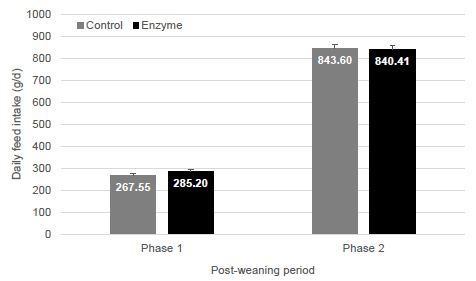
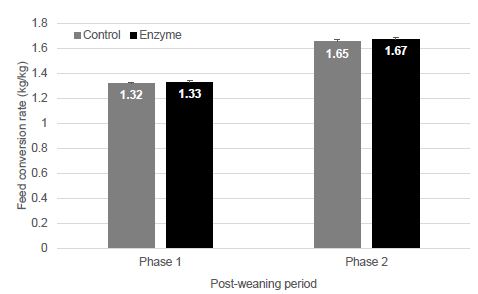
| Disease |
Infectious agent. Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Actinomycosis |
Actinomyces israelii and others of the same genus
Chronic disease located in orocervical facial,, thoracic and abdominopelvic regions |
Sporadic throughout the world |
A42
rare disease small impact |
low |
Frequency maximal between 20 and 60 years of age. Mucosal barrier disruption caused by surgery or irradiation, and immunocompromising conditions. |
Not demonstrated |
N/A |
humans |
Reasonably common component of oral flora. Can cause problems following trauma that allows access. Prolonged administration of penicillin can be effective. No spontaneous recovery. |
| Anthrax, Woolsorter disease, ragpicker disease. |
Bacillus anthracis. Three forms depending on route if introduction. Cutaneous, inhalation, gastrointestinal occasionally among drug users. |
S and Central America, S and E Europe, Asia and Africa |
A22
Primarily a disease of herbivores |
Uncertain |
A zoonosis. An infrequent or sporadic disease among veterinarians, wild life workers and agricultural workers. |
Second attacks rare. Immunisation for individuals at high risk because of their location or occupation using a cell free isolate is said to be effective. |
For animals and those humans at occupational risk |
Animals and viable spores can persist in soil for decades |
Widely bruited as a weapon for terrorism though it has probably not yet been deployed. Complex regimens of post exposure prophylaxis are deployed. |
| Bartonellosis (Oroya fever, Verruga Peruana, Carrion Disease) |
Bartonella bacilliformis
Either a life threatening febrile anaemia (Oroyo fever) or a benign dermal eruption (Verruga Peruana). There are many spp. Of Bartonella with much more complex patterns of infection than shown in the manual. See also Cat Scratch fever and Trench Fever. |
Mountain valleys of Peru, Ecuador and Southwest Columbia between altitudes of 2000 and 9,200 feet where sand flies are present |
A44.0, A44.1
Mortality with untreated oroya fever can be as high as ninety per cent |
General |
More severe in adults than in children. Most common in tourists, i.e. immunologically naïve individuals. |
Inapparent infections and carriers are known(up to 5% in endemic areas). Recovery from untreated Oroya fever almost invariably leads to permanent immunity though the Verruga stage may recur. Asymptomatic infections and a carrier state are known. |
N/A |
Humans, no known animal reservoir. Vector sand flies. |
Treatment with antibiotics can be partly successful. |
| Intestinal Botulism, Infant Botulism |
Clostridium botulinum is the source of botulinum neurotoxin that causes the disease. Other spp of the genus can be involved. Severe neuropathogenic disease. Respiratory failure common cause of death. |
Worldwide, sporadic, family and general outbreaks are associated with imperfect food preparation |
A05.1 cumulative cases world wide of which 1400 were from the USA. Nevertheless regarded as a major hazard presumably because of its high lethality. |
General |
Almost all hospitalised patients were between two weeks and one year of age. 94% were less than six months!. Adults with bowel problems or treated with antibiotics can be at special risk. The use of botulinum toxin has been associated with the development of iatrogenic disease. |
? |
Antitoxin is administered presumably as a passively given antibody. There seems to have been no attempt at active immunisation. |
Spores in soil ubiquitous |
In effect a disease of the food industry. Most problems it is written are caused not by ingestion of preformed toxin but of bacterial spores that germinate to give rise to more organisms that secrete the toxin. Important as a potential bioterrorism tool. I.V. antitoxin |
| Disease |
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Brucellosis, undulant fever, Malta fever, Mediterranean fever |
Brucella abortus in a variety of strains
A systemic disease. Very complex pattern of symptoms |
Worldwide |
A23
Less now than heretofore. Incidence in USA 120 current cases. In other parts of the world probably unreported and undiagnosed. |
Severity and duration of clinical illness subject to wide (unexplained) variation. |
A disease of those working with farm animals as abattoir, vets or direct contact with animals. Persons eating uncooked meat are at higher risk. |
Unknown |
None in man but active successful immunisation of cattle is practised. |
Cattle, swine, goats and pigs. |
It has been suggested that infection with Brucella was a negative indication for cancer. Equally a number of suggestions have been made that brucella antigens could help suppress cancer. The evidence so far is slender. Anti Biotics |
| Campylobacter enteritis, Vibrionic enteritis |
Campylobacter jejunis
Diarrhea. |
World wide |
A04.514% of diarrhoea worldwide caused by these organisms. |
Many infections are asymptomatic |
Children under five and young adults are at higher risk. Immunocompromised individuals at higher risk. |
|
None |
Poultry and cattle mainly but many other animals. Most raw poultry meat contaminated! |
Common disease with considerable impact. Treatment not generally indicated! Rehydration and electrolytes. |
| Cat Scratch Disease, benign lymphoreticulosis |
Bartonella benselae
Subacute usually self limiting disease. Affecting lymphoid system and often causing fever |
Worldwide but uncommon |
A28.1
Slight |
unknown |
Immuno-compromised host most infected but some evidence that younger children and younger adults are more affected |
Diagnosis sometimes based on serological evidence of anti-Bartonellaantibody. |
None |
Domestic cats. There is no evidence of adverse effects on cats even when they are bacteraemic |
Interesting example though too little is known about it to place much weight on it. The fact that infected cats are asymptomatic is noteworthy. Antibiotics |
| Chancroid, ulcus molle, soft chancre
|
Haemophilus ducreyi
STD |
Sporadic. Less in temperate regions |
A57
small |
, no natural resistance, the circumcised are at less risk than the uncircumcised. |
Men who frequent prostitutes! |
None recorded |
None |
Humans |
Unpleasant condition with too little known Antibiotics |
| Chlamydial infections, Genital (psittacosis and respiratory disease dealt with separately) |
Chlamydia trachomatitis
STD |
Common |
A56
Nuisance rather than major threat |
General,majority of infected women are asymptomatic, up to 25% of infected males the same. |
None given |
No acquired immunity has been demonstrated repeated infections common. |
N/A |
humans |
Common, seems to have little if any impact on immunological mechanisms. Antibiotics can render patients non-infectious. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Vibrio parahemyticus infection |
Vibrio parahemolyticus
Enteritis |
Sporadic in many parts of the world. Marine coastal environments |
A05.3
A disease of moderate severity. Rarely systemic or lethal |
Various anterior medical conditions such as liver disease, decreased gastric acidity or immunosuppression. |
oysters |
No indication given |
None |
Marine silt |
Rehydration, antibiotics |
| Vibrio vulnificus |
Vibrio vulnificus.
Septicaemia commonly but other symptoms encountered. |
Marine environments particularly but not exclusively in N. America. |
A05.3
Septicaemia fatal in 50% of casess |
Characteristically in patients with chronic liver disease, alcoholism, hemochromatosis or immunosuppression. |
Oysters. Sea water exposure of open wounds. |
No indication given |
None |
Free living organism in estuarine environments. Uncooked sea food can be source of infection |
Rehydration, antibiotics |
| Cholera (serotypes other than 01 and 0139) |
Vibrio choleraEnteritis and otitis media, and cellulitis. |
2-3% of cases of diarrhoea (including travellers) in tropical countries |
A05.81Small relative to the pathogenic 01 and 0139 serotypes |
All humans said to be susceptible |
Wound infections, malnutrition and immunosuppression |
NK |
N/A |
brackish waters where they are part of the normal flora |
associated with outbreaks of enteritis. Fluid replacement used. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Bacterial conjunctivitis, pink eye, sticky eye, and Brazilian purpuric fever. |
Many organisms can be involved the most important are Hemophilus influenzae and Streptococcus pneumoniae. Viral causes dealt with under Viral Disease heading.
Eyes.
|
Widespread and common |
A48.4
Warmer climates seasonal epidemics in the main non fatal but systemic fatal disease has been reported (Brazilian purpuric fever) |
Probably general |
Children under five most affected. The debilitated and the aged are particularly susceptible to staph. Infection. |
Low grade after infection and varies with the infectious agent Clearly lack of knowledge here |
N/A |
Humans |
Sulfacetamide plus or minus antibiotics |
| Chlamydial conjunctivitis, inclusion conjunctivitis, paratrachoma |
Chlamydia trachomatis
Eyes. An STD |
Sporadic throughout the world. |
A74.0
|
? |
Affects new born infants in particular otherwise a complication of genital infection in adults |
No evidence of resistance to reinfection though severity of disease is variable |
N/A |
Humans |
Often acquired by infants during birth process. Antibiotics |
| Diarrhoea caused by E. coli, Enterohaemorrhagic strains. Shiga toxin producing strains. complex of pathogens |
STEC initially Intestine but can create massive renal and other potentially lethal problems. |
Important problems in N America, Europe, S Africa, Japan Australia. |
A04.3 Outbreaks associated with a variety of poorly cooked foods |
infectious dose is very low. Little is known about susceptibility or immunity |
Old age, achlorhydria and infants under five.
Diabetics and infants of infected mothers. |
None reported
|
none |
Cattle and perhaps deer, more rarely humans |
Fluid and electrolyte replacement. Antibiotic treatment uncertain and potentially dangerous. |
| Diarrhoea caused by E. coli, Enterotoxigenic strains. |
ETEC |
Primarily in developing countries |
A04.1A major cause of travellers diarrhoea. In developing countries multiple infections of infants occur |
Probably universal though it is not so stated |
Less frequent in adults. Children <4years of age in developing countries can have up to 32% mortality. WHO reports up to 380,000 deaths of such children annually. Contaminated food particular risk factor. |
Serotype specific immunity is acquired following infection. Problem is that there are so many serotypes |
none |
humans |
Fluid replacement, rehydration salts. Anti-microbial agents often deemed dangerous. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Diarrhoea caused by E. coli, Entero-invasive strains |
EIEC |
Endemic in developing countries |
A04.2
Cause about 1-5% of cases visiting treatment centres |
NK |
Visitors and children in endemic regions |
NK |
None |
Humans |
Fluid replacement. Few centres treat this somewhat more rare disease. |
| Diarrhoea caused by E. coli, enteropathogenic strains
|
EPEC |
Oldest recognized form of largely infant diarrhoea, largely disappeared from the Western world.. |
A04.0
Still a major problem in many other places in the developing world where fatality rates can be high |
Susceptibility is confined to young infants but why is not known. It could be immunity but that is not established. Experiments on adults suggest that immunity is the answer. |
Disease uncommon in breast fed infants. Often associated with contaminated infant formula. Outbreaks due to contaminated water or rice have been reported. |
Likely but not certain |
None |
Humans |
Fluid replacement |
| Intestinal E. coli infections and others |
EAEC,, DAEC |
Cause of sporadic out breaks associated with acute and persistent diarrhoea in infants. In developing and developed countries. |
A04.4
EAEC can be a cause of traveller’s diarrhoea in as many as 20% o cases in some reports. |
DAEC in some reports more pathogenic in children but information is sparse. |
Contaminated food and drink. |
Infants in particular are susceptible. |
NK |
Likely humans possibly animals |
Rehydration treatment and anti-microbials are said to be useful. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Diphtheria |
Corynebacterium diphtheria
Mucous membranes of upper respiratory tract, more rarely other mucous membranes. A potent exotoxin causes the problems. Carried by some strains of Corynebacteria themselves infected with the toxin generating phage. |
A disease of colder months in temperate climes |
A36
Major outbreaks have occurred in number of areas of the world in recent years in unvaccinated individuals |
Not stated |
Infants born to immune mothers are protected for up to six months by passively acquired antibody. |
Lifelong immunity is usually but not always acquired after infection. Immunisation with toxoid also produces lifelong immunity (non toxigenic bacteria rarely cause disease) |
Very effective |
Humans |
Presence of a phage as is true for some other bacterial spp dictates capacity to produce a toxin that is the main cause of pathogenesis. Anti toxin + sometimes antibiotics |
| helicobacter pylori infection |
Helicobacter pylori
Causes acute and chronic gastritis. |
Worldwide Said to be present in 50% of the human population |
K29 usually no symptoms but for some gastritis and gastric Carcinoma can follow infection |
Universal it is supposed. Increasing prevalence with increasing age. |
Not identified but supposed that there must be identifiable risk factors. Lower socioeconomic status appears to be associated with higher prevalence. |
None recognized |
None presently available |
Humans probably though it has been found in other primates |
Treatment with antibiotics can be successful in reducing gastritis stopping continuation to malignancy. Controversial antibiotics |
Ehrlichiosis,
Anaplasmosis, Senetsu Fever, Neoehrlichosis |
Ehrlichia sennetsu
Anaplasma cytophylum
Neorickettsia senesu,
Neoehrlichia misurensis
Acute febrile illnesses with small intracellular bacteria that survive inside a variety of phagocytic white blood cells. |
Four diseases here one Sennetsu fever the other three different forms of ehrlichiosis.. Distribution mainly in north and south America, Europe, Western Japan and Malaysia |
A79.8
Range from mild illnesses to severe life threatening disease. Diagnosis tricky to differentiate it from a wide variety of viral illnesses. |
General |
Older, debilitated or immunosuppressed people more susceptible |
NK |
Re-infection rare. implication is derived adaptive immunity. Consumption of raw fish suspected cause with Senetsu fever |
Not certain but a variety of vertebrate hosts are involved. Ticks can be vectors. |
Doxycycline |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Gonococcal infection, (a separate category of gonococcal conjunctivitis is here ignored), clap, strain, gleet, dose, G.C. |
Neisseria gonorrhoeae
STD particularly in the commercial sector.
|
Common worldwide |
A54.0 A54.2, Rarely lethal but with many unpleasant symptoms |
General |
Not given |
Humoral and secretory antibodies have been demonstrated but the bacterium is antigenically heterogeneous and reinfection is common |
none |
Strictly a human disease |
Major STD. Antibiotics but many resistant plasmids exist. |
| Granuloma inguinale (Donovan osis) |
Calymmactobacterium granulomatis
Genitalia in 90% of cases |
Rare in industrialised countries but even there small occasional epidemics are recorded. |
A58
Slight fortunately but cluster outbreaks have been recorded in tropical and semi-tropical countries |
Most common in 20-30 year old males but known also in 1-4 year old children and it is suggested that non-sexual transmission can occur. |
Bought sexual activity |
None it appears, ie second attacks occur (presumably after treatment of the first attack) |
none |
Humans |
Horrid condition not easily brought under control that essentially erodes the genital regions. We are clearly short of information on the disease. Antibiotics |
| Legionellosis (there is also non pneumonic legionellosis, Pontiac fever, which is here ignored). Legionnaires disease |
Various legionellae |
Widespread but sporadic more common in summer and autumn |
A48/1
Regarded as dangerous case fatality rate can be 15%. |
Age related |
Males more than females usually in patients over 50 years of age. Patients who smoke or who have diabetes mellitus are at special risk. immunocompromised people especially those on corticosteroids special. Infected cooling towers and warm but not hot water |
Iimplication is that there is an immune response in that in a few locations antibodies have been detected in 1-20% of the general population. |
None stated |
Aqueous primarily, hot water systems not properly maintained. |
Why is it called legionnaires disease? Some antibiotics are effective. Disinfection of suspected water supplies is effective. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Leprosy (two forms tuberculoid and lepromatous of which the latter is more severe), Hansen’s disease. |
Mycobacterium leprae
Cannot be grown in culture.
Chronic disease of the skin and peripheral nerves. |
Chief endemic areas are S and S Eastern Asia, Indonesia, tropical Africa and parts of latin America. |
A30
Considerably over a million cases worldwide but it should be stressed that probably only a small proportion of those infected develop symptoms. |
Rate of lepromin positive tests increases with age but as this can give false positives it is not clear that this represents a build up of asymptomatic infections. |
In childhood, rarely seen under three. (not long enough for the disease to develop?) Incubation time can be anything from nine months to twenty years! |
Immunity said to depend on a cell mediated response though antibodies are produced. It is argued that 95% of the population are naturally immune. In the manual this is termed innate immunity but this terminology is incorrect. |
BCG may have some use in this context! |
Humans and armadillos. |
Still a major disease not easily cured. Prolonged treatment with a variety of antibiotics but resistance is a problem. |
| Leptospirosis, Weil’s Disease, Swineherd fever, mud fever, Haemorrhagic jaundice and other names. |
Organisms from the genus Leptospira . Large number of serotypes.
First phase of infection can be high fever. Second phase coincident in time with development of antibodies Recovery of untreated cases can take several months. |
Worldwide except polar regions. Most prevalent in tropical and sub-tropical regions. |
A27
Asymptomatic or mild infections are common but occasional epidemics have killed many of those infected. In general 5-10% of cases progress to severe illness. |
general |
Case fatality rate is generally low but can reach twenty percent in those with renal damage. Largely an occupational disease for those working in sugar plantations and rice fields Often a disease of bathers and campers no particular other predilections given |
Serovar specific immunity arises |
In both workers at risk and the local domestic animals this has been attempted. The results are either not known or simply not given |
Wide variety of wild and domestic animals. |
Prompt specific, early treatment with antibiotics can be effective. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Listeriosis |
Listeria monocytogenes
Can present as an invasive disease with septicaemia and meningitis. |
An uncommonly diagnosed infection in USA but frequency elsewhere in the world not given. Outbreak cases are reported associated with contaminated food. |
A32
Accounts for a small fraction of all blood borne diseases. Despite this it is regarded as an important cause of severe illness. |
Most children and young adults are resistant |
Adults over the age of 40 become more sensitive and Almost all the debilitating and immunosuppressive conditions, (including pregnancy) confer heightened sensitivity |
NK |
None |
Solid forage water mud and silage plus domestic animals and asymptomatic human (fecal) shedders. |
Commonly associated with manufacture of soft cheeses of which it is part of the bacterial array. Antibiotics work |
| Lyme disease, Lyme borreiosis, tickborne meningopoly neuritis |
Borelia burgdoreferi and others
Distinctive skin lesions and a variety of other systemic manifestations over a long time period. |
Found in many places particularly well known in USA but also in Europe, China and Japan |
A69.2, L90.4Difficult to say on evidence presented. Clearly an uncomfortable and chronic disease that can usually be successfully treated, |
Universal apparently |
None stated |
Re-infection has occurred in those treated early with antibiotics, the implication is either that immunity is not a result of infection or that antibiotic treatment prevents the development of lasting immunity. |
Vaccines have been developed and used with up to 76% success but this is not a clear story. |
Disease is maintained in an enzootic transmission cycle that involves ixodid ticks wild rodents and deer. |
A zoonosis Treatment with antibiotics |
|
|
|
|
|
|
|
|
|
|
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Lymphogranuloma venereum, climatic or tropical bubo |
Chlamydia trachomatis genotypes.
STD in both sexes. In men who have sex with men proctitis can develop. |
Worldwide especially in the tropical and subtropical areas |
A55
Disease untreated is debilitating but not usually fatal. |
General |
Male homosexuals |
Not clear |
None |
Humans often asymptomatic females. |
Antibiotics can be effective. |
| Melioidosis, Whitmore disease
Glanders |
Burkholderia pseudomallei causative agent for Melioiodosis, Burkholderia mallei for Glanders.
Cutaneous or visceral abscesses with subsequent development of a wide range of potentially lethal systemic symptoms. |
A significant cause of community acquired sepsis in the tropics. |
A24.1, A24.4, A24.0
In a number of largely tropical places |
Problem here of definition. Disease is uncommon even in parts of the world where the infective organism exists and the (rural) population are in frequent contact with the soil in which the bacterium exists. The implication is that infection as a common disease is rare |
Those with abraided or burned skin who also have intimate contact with soil |
Not clear. Change of environment, eg development of diabetes mellitus can give recrudescence of what is probably a long term latent infection |
None |
Soil and water. A saprophyte. Various animals can become infected but there are no known vectors to which they transfer the organism but they can spread it around passively |
TMP-SMX is effective treatment ( a mixture of trimethoprim and sulphamethoxazole) |
| Meningitis, cerebrospinal fever |
Neisseria menigitidis various strains/serotypes exist that define different epidemics
Inflammation of the meninges is the defining feature of this disease and here three bacterial causes of the condition will be considered. A petechial rash often present in Europe and N. America but rarely in Africa. |
Ubiquitous |
A39.0
Nowadays in developed country case-fatality rate is 8-15%. 5-10% of those in endemic countries may be asymptomatic carriers of whom very few progress to disease. |
Susceptibility to disease is low and decreases with age. Disease is primarily of very young children and young adults. More common in males than in females. Highest burden of diseasein African meningeal belt. |
Splenectomy, certain complement components |
Group specific immunity of unknown duration follows even subclinical infection |
Dead vaccines are available and have been reasonably successfully applied |
Humans |
Epidemics tend to crop up in those inhabiting crowded communal quarters. A variety of antibiotics can be effective treatment. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Hemophilus meningitis, |
Hemophilus influenza In industrialised countries before widespread use of Hib conjugate, vaccines meningitis was the most common presentation, epiglottitis and bacteremia were the next most common. In developing countries lower respiratory tract infection was the most common first symptom. Pneumonia of this kind has been said to cause 480,000 deaths per year among children under five years of age. |
Worldwide |
G00.0
Most prevalent among children three months to three years. Vaccine use has cut down the disease in the USA and a higher proportion of cases is now seen in adults |
Universal |
Age |
Immunity usually associated with presence of circulating anti-capsular antibodies acquired transplacentally or by immunisation. Or a prior infection |
Yes with polysaccharides |
Humans |
Antibiotics but resistance is now a problem. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Nocardiosis,
Actinomycetoma |
Nocardia asteroidsand others of that ilkPulmonary infection |
Occasional sporadic disease in all parts of the world |
B47.1
Difficult to say on evidence presented |
Unknown |
Endogenous or iatrogenic adrenal hypercorticism and probably primary alveolar proteinosis |
Opportunistic infection can occur in immunosuppressed individuals. Implication is that there is an immune mechanism. |
None |
A saprophyte found in soil, water and organic material. |
TMP-SMX depending on serotyope specificity. |
| Pertussis, Whooping cough
Parapertussis, a milder version of the disease |
Bordetella pertussis
Bordetella parapertussis.
A respiratory disease with occasional systemic complications. |
An endemic disease common especially young children everywhere |
A37.0, A37.9
A37.1Schemes of immunisation have reduced the prevalence. This disease is among the most lethal of all the childhood diseases in unimmunizd individuals. In recent years it is increasingly recognized in older children and adults even when they have been immunized as infants. |
Universal among non-immunised individuals. Milder and atypical cases occur in all groups (the hundred day cough!) |
Malnutrition and enteric infections can be predisposing conditions. Interestingly, transplacental transfer of immunity has never been demonstrated. Note comment in Vaccines column. |
One attack usually confers prolonged immunity although second attacks can occur. |
A killed vaccine is widely used. Maternal antibodies are carried across the placenta which observation has led several countries to adopt immunisation prior to pregnancy but whether this stratagem works is not stated. |
Humans |
? Erythromycin reduces the period of communicability but does not affect symptoms except when given very early. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Pinta, Carate |
Treponema caroteum a spirochaete. A chronic non venereal skin disease. |
Found only among crowded rural populations living in poor conditions in the American tropics |
A67
Physical disability does not occur. Organ systems are not involved Not fatal. Said to be on its way to eradication! |
Not defined presumably as in other treponematoses (various syphilitic diseases in relation to which immunity can develop) |
Mainly a disease of children |
Not stated |
None |
Humans. Various biting flies are suspected of being vectors. |
A none venereal disease. Antibiotics fix it. |
| Plague, Pestis |
Yersinia pestis
Three presentations, bubonic, pneumonic and septicemic. |
Almost everywhere that there are wild rodents.
Foci of infection exist in the Americas particularly in N. Eastern Brazil. |
A20
Both bubonic and pneumonic forms can be lethal and in the past have been responsible for major epidemic mortality. Untreated the mortality rate is 50-60% These days it is clearly less of a problem than it was. |
General |
None given |
Some immunity after recovery. |
Yes both living and dead. They can be efficient for about three months |
Wild Rats with their fleas as the vector. |
A potential terrorist weapon. Streptomycin is the drug of choice.. The pathogenicity is associated with a mutation. P. Unusual example of a mutation conferring increased pathogenesis. |
|
|
|
|
|
|
|
|
|
|
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Pneumonia, There are many organisms which can cause pneumonia. Here only four will be dealt with 1,pneumococcal pneumonia |
Streptococcus pneumoniae (twenty three capsular types account for ninety per cent of the infections that cause bacterial pneumonia in the USA)
Often sudden onset high fever with a wide variety of complications. |
Essentially worldwide though increasing control was being developed. Now resistance to antibiotics is becoming a problem. |
J13
A major cause of death in developing countries among new born children. The disease can be associated with influenza infection. |
General in the sense that I suspect the organism concerned is always present. Not general in the sense that only few get the disease! The definition of susceptibility is here strained. |
In young infants the mortality even using antibiotics can be 60%, malnutrition and low birth weight are contributory factors. Any harm to the lower respiratory tract is predisposing. In adults almost any co-morbidity can predispose. |
Serotype specific immunity can be long lasting. |
A vaccine with all 23 capsular types is available, it is not effective in children under the age of 2 but it can be useful Prophylaxis in the elderly |
Humans (many normal individuals have the organism concerned as part of their respiratory tract flora.) |
Splenectomy is a predisposing factor.. Antibiotic resistance now common. |
| Pneumonia, primary atypical pneumonia |
Mycoplasma pneumoniae
The taxonomic designation of this organism is uncertain being either virus or bacterium. Here it is included among the bacterial causes of the symptom.
|
Worldwide sporadic and epidemic |
J15.7
Fatalities rare, differential diagnosis difficult there being at least ten other infectious causes of pneumonia! Clinical disease occurs in 3-30% of infections |
Susceptibility not mentioned! |
None given |
Second infections do occur. Immunity correlated with antibodies that can remain for a while |
None |
Humans |
Impression given is of an occasional infection that elicits only little immunity perhaps because the causal organism simply does not really like it in man. There are many species that infect domestic animals but there is no record here of zoonotic infection. Antibiotics work. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Pneumonia, neonatal eosinophilic pneumonia, Congenital pneumonia due to Chlamydia |
Chlamydia trachomatis particular immunotypes
A sub-acute pulmonary disease
|
Probably coincides with the worldwide distribution of the causative organism as a genitally transmited infection |
P23.1
Illness usually moderate |
? |
Infants born to mothers who have chlamydial genital infection. |
Unknown. Maternal antibody is not protective |
None |
Humans |
Oral erythromycin. |
| Pneumonia
pneumonia due to Chlamydia |
Chlamydia pneumoniae
An acute respiratory disease. |
Presumably worldwide |
J16.0
Death rare in uncomplicated cases |
Universal |
Increased likelihood of clinical disease with pre-existing chronic disease. |
Some suggestion of immunity after infection however second episodes of pneumonia are not unusual |
None |
Humans probably |
Oral tetracyclines |
| Psittacosis, Ornithosis, Parrot fever, Avian Chlamydiosis. |
Chlamydophila psittaci
An acute disease with systemic presentations and respiratory symptoms. |
World wide |
A70
Usually mild |
Universal |
Exposure to birds and old age |
Immunity after infection incomplete and transitory |
none |
Parakeets, parrots and love birds mainly. Birds that appear healthy can become shedders under conditions of stress. |
|
| Q fever, Query fever. |
Coxiella burnettii
An acute febrile disease. Various complications sometimes involving the liver. |
Worldwide, under reported It should be noted that fatality in untreated cases can be as high as 2.4%. |
A78
|
General |
A variety of occupations particularly veterinarian and abattoir workers. are associated with this disease. |
Immunity probably lifelong after recovery from disease. Cell mediated immunity lasts longer than humoral (does the organismpersist?) |
Not commercially available but for those at high risk vaccines that are effective have been prepared. |
Sheep, cattle., goats and dogs. |
Tetracyclines for acute disease Antibiotics. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Relapsing fever, |
Borrelia recurrentis
Fever often recurrent. |
Worldwide except Australia and New Zealand |
A68
Untreated case fatality can be 2-10% |
general |
None stated |
Unknown but second attacks are rare. |
None |
Humans and wild rodent. There are some differences between the tick and louse borne forms of the disase. |
Tetracyclines |
| Rickettsioses, tick borne, rocky mountain spotted fever.
Some twelve fevers are recorded under this heading from specific geographical locations, here only two will be dealt with |
Rickettsia rickettsii |
Throughout USA and some S American states |
A77
Case fatality 13-25% if not recognized and treated |
general |
Patients older than 40 |
Immunity not stated in 20th Edition. In earlier editions it is stated that one attack confers life time immunity. |
none |
Maintained in nature by ticks can be transferred to for example dogs in which infection is usually subclinical |
tetracyclines |
| Rickettsioses, tick borne, Boutonneuse fever |
Rickettsia conori and related organisms. |
Widely distributed in Africa and India and Eastern Europe |
A77.1
Mild to severe febrile illness |
General |
Travellers! |
Immunity not stated in 20th Edition. |
None |
Ticks and dogs (travellers dogs pick up infected ticks that are taken home with the owners who subsequently acquire the infection. |
Tetracyclines |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Salmonellosis, |
Salmonella bongori and S enterica
More than 2000 serotypes are recorded.
Severe enteritis |
Worldwide, can occur in massive epidemics |
AA02
million cases reported annually in the USA alone! Not usually fatal |
General, severity of condition related to ‘dosage’ of infection. |
The very young, achlorhydria, AIDS patients, malnutrition and other debilitating conditions. |
None recorded |
None available |
Predominantly an infection of food but commonly carried by a wide variety of animals. |
A major disease the only causes problems in high concentrations No treatment generally indicated except rehydration. In the very young and very old antibiotics can be given. Patients with AIDS may require lifelong therapy |
| Shigellosis, bacillary dysentery |
Shigella various spp
Distal small intestine and colon.
|
Worldwide |
A03
Estimated that shigellosis causes14,000 deaths annually but mild and asymptomatic infections occur and the illness is usually self-limiting |
General, infection can follow ingestion of a small no of bacteria. |
Very young and the elderly and debilitated patients of many kinds. Breast feeding is protective for young infant. Homosexual men where conditions are poor such as in jails. |
Not recorded. Secondary attack rates can be up to 40% in specific households. |
Vaccines with some short term efficacy have been deployed. There is a clear need for an effective long term vaccine. |
Humans |
A major disease with far too little said about it. Particularly the issue of immunity is not addressed perhaps because there is not any although the experimental vaccines have had some success. Symptomatic treatment except in severe cases. Antibiotics can then work but there are major and complex problems with resistance. |
| Staphylococcal diseases in the community, boils, carbuncles,, sepsis, infected lacerations. |
Staphyllococcus aureus various coagulase positive strains are involved, identified
Skin |
Worldwide, highest incidence of disease where standards of hygiene are lowest. |
L02, B95.6, B 95.8 A41.0 A 41.2
|
Universal. 20-30% of general population are nasal carriers of the relevant organisms. Auto-infection responsible for at least two thirds of infection with disease. |
Newborn and all sorts of generally debilitated patients |
Immune mechanisms said to depend on the instruments of innate immunity. |
None recorded |
Humans more rarely animals |
Local disease does not warrant treatment. Treatment of systematized infection with antibiotics is undertaken. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Staphylococcal diseases in the community, boils, carbuncles sepsis, infected lacerations. |
Staphyllococcus aureus various coagulase positive strains are identified.
Skin |
World wide, highest incidence of disease where standards of hygiene are lowest. |
L02, B95.6, B 95.8 A41.0 A 41.2
|
Universal 20-30% of general population are nasal carriers of the relevant organisms. Auto-infection responsible for at least two thirds of infection with disease. |
Newborn and all sorts of generally debilitated patients |
Immune mechanisms said to depend on the instruments of innate immunity i.e. not adaptive. |
None recorded |
Humans more rarely animals |
Local disease does not warrant treatment. Treatment of systematized infection with antibiotics is undertaken. |
| Staphylococcal diseases, in hospital nurseries, impetigo neonatorum, scaled skin syndrome, abscess of the breast |
As above
Impetigo
Skin. |
Worldwide exacerbated by laxity in hygiene precautions and emergence of antibiotic resistance. |
L 01
Big problem quantification of it is given |
In the new born susceptibility seems to be universal |
Infected infants remain at risk for the duration of infection with a pathogenic strain. |
? |
None |
As above |
Antibiotics for both local and systemic infections can be effective. |
| Staphylococcal diseases, in medical and surgical wards. |
As above plus the problem that 90% of the strains causing problems are antibiotic resistant (MRSA).
A wide variety of conditions including endocarditis, osteomyelitis, pneumonia, meningitis, |
|
J15.2, M86, M00.0, 133.0.
Probably the most serious problem of hospitals that have surgery, implants and so on. |
Universal? |
Any sick people |
? |
None |
As above |
The organism concerned is essentially ubiquitous and seems on the face of it to elicit little or no immune response Appropriate antimicrobials with great problems of resistsnce.. |
| Streptococcal infection, caused by group A Hemolytic streps, a large no of diseases including scarlet fever, sore throat, erysipelas, puerperal fever, rheumatic fever necrotising fasciitis and so on. |
Streptococcus pyogenes group A
A wide variety of conditions mimicking sometimes the conditions caused by Staphylococci. |
The diseases concerned need separate treatment as they differ in distribution across the world. |
Again treatment of the diseases as one category is not easy for example rheumatic fever is much less than it was but in 1985 there were outbreaks in the USA. The highest incidence of impetigo occurs in young children in the late fall and so on. |
General |
None quoted |
For some of the diseases long lasting type specific immunity follows infection for others it does not. For example rheumatic disease has a significant risk of recurrence. It seems that although we are dealing with the same basic organism its many disease manifestations are relatively ill understood |
None |
Humans |
It seems extraordinary that such a common set of diseases should be lumped together despite clear differences between them in terms of mechanisms of disease. Antibiotics. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Streptococcal infection, caused by group A Hemolytic streps, a large no of diseases including scarlet fever, sore throat, erysipelas, puerperal fever, rheumatic fever necrotising fasciitis and so on. |
Streptococcus pyogenes group A
A wide variety of conditions mimicking sometimes the conditions caused by Staphylococci. |
The diseases concerned need separate treatment as they differ in distribution across the world. |
Again treatment of the diseases as one category is not easy for example rheumatic fever is much less than it was but in 1985 there were outbreaks in the USA. The highest incidence of impetigo occurs in young children in the late fall and so on. |
General |
None quoted |
For some of the diseases long lasting type specific immunity follows infection for others it does not. For example rheumatic disease has a significant risk of recurrence. It seems that although we are dealing with the same basic organism its many disease manifestations are relatively ill understood |
None |
Humans |
It seems extraordinary that such a common set of diseases should be lumped together despite clear differences between them in terms of mechanisms of disease. Antibiotics. |
| Streptococcal infection, caused by group B, streptococcal sepsis of the new born. (and dental caries of the new born), baby bottle tooth decay. |
Streptococcus aagalacticae
Serious invasive diseases of the newborn.
Same as above except group B |
|
P36.0
Thought to occur worldwide. Information here lacking.. Most studies from N. America and Europe |
|
Babies born prematurely, particularly when there is rupture of the membranes more than 18 hours prior to delivery. |
|
A vaccine for pregnant women to stimulate antibody production to restrict invasivc disease is said to be under production. |
Hunans. Commonly found in GI and urinary tracts. |
Anti microbial preparations. Given particularly to infected pregnant women prior to labour. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Syphilis. |
Treponema pallidum
STD. Extremely complex disease with three distinct phases. |
Widespread among sexually active individuals |
A50-52 Extremely nasty consequences can arises in untreated chronic disease although ilatency is known. |
Universal although only 30% of exposures result in disease.. |
Immunosuppression, particularly HIV |
Immunity to re- or further- infection usually develops in time but paradoxically it often fails to develop because of early treatment.. |
None available |
Humans |
Complex treatment protocols often involving penicillin |
| Tetanus, lockjaw, Obstretrical tetanus, tetanus neonatorum. |
Clostridium tetani
Acute disease caused by an exotoxin. A variety of disease forms can emerge after contact with the causal organism. |
Worldwide |
A35, A 33. A.34.
Relatively uncommon in industrialised countries case fatality 2.3% for those aged under 20-39 and 18% for those over 60. Case fatality can up to 80% depending on quality of care. |
General |
Infants and elderly at higher risk. Members of service groups such as armed forces and police and those in contact with sewage. |
Paradoxically recovery from infection does not guarantee immunity and there is no detectable antibody (this is somewhat of a paradox in that anti-toxin immunisation is effective for long periods of time). |
Active long lasting immunity is elicited by toxoid |
Intestines of cattle and soil in which human and or animal faeces are found. The organism is essentially everywhere. |
Prohylactic antibiotics in those by culture felt to obe at risk |
| Trachoma, |
Chlamydia trachomatis, specific serovars.
Initially conjunctivitis,
Can resolve spontaneously but repeated reinfection can lead to blindness. |
Worldwide occurring as an endemic disease largely in poorer communities |
A71
A major cause of development of blindness over a long period of time. |
General. Active disease tends not to be seen in older children and adults |
Poor living conditions,
Dust and fine sand may exacerbate the condition. |
No evidence for immunity |
None successful |
Humans |
A major disease but seemingly curable. Topical tetracylines can be effective. Repositioning of eyelashes so they no longer abrade the cornea can be effective. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Trench fever, Quintana fever |
Bartonella quintana
A typically febrile non fatal septicaemia.
|
Scattered but in many places |
A79
Particularly prevalent in world war one trenches |
General |
Immunocompormised patients have a variety of severe symptoms |
unknown |
None recorded |
Humans but vector is the body louse. |
Tetracyclines |
| Tuberculosis, TB
|
Mycobacterium tuberculosis and to far lesser extent M. bovis.
It is estimated that 1/3 of the world population is presently infected. Active disease can be pulmonary or extra pulmonary.
There is given in the manual a brief account of non-tuberculous mycobacterial disease. Here it is not considered. |
Worldwide |
AA15-19
Probably the biggest single cause of mortality and disability associated with infection. Despite this it is likely that the majority (90%) of those infected enter a latent condition from which there is always a danger of reactivation. |
Ostensibly risk of infection is related to degree of exposure. The first six to twelve months after infection are the most dangerous for development of full blown disease. |
Risk of developing disease highest under the age of 3, lowest in later childhood and high again among young adults the aged and the immunosuppressed HIV in particular. Other debilitating diseases can contribute to the likelihood of reactivation. |
The utility in some circumstances of BCG vaccination suggests that there is a degree if immunological control but one wonders if this is niche occupation rather than immunity per se. There is little in the text about immunity except in relation to the PPD skin testing that is usually positive in infected people. This is a very complex story |
BCG has been deployed but in some circumstances it seems not to work in terms of avoiding disease. The whole issue is complicated by the differences in frequency of wild type challenge in some of the regions that are being compared. |
Humans primarily. The argument in relation to badgers and cattle still rages. |
Antibimicrobials. Whether latent TB should be treated seems not to have been addressed. Also the latent status seems to be little understood |
|
|
|
|
|
|
|
|
|
|
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Tularaemia, Rabbit fever, Deer-fly fever, Ohara disease, Francis disease. |
Francisella tularensis
Skin and lymphadenopathy, or the latter without the former. |
N America, former Soviet Union China and Japan |
A21
Complex set of diseases with a wide variety of symptoms.. |
All ages susceptible, and presumably all people |
Closely linked to occupational and recreational activities. |
Long term immunity follows recovery from infection. |
None |
Numerous wild animals, with a tick vector usually or, less commonly, deer fly |
Streptomycin. |
| Typhoid, paratyphoid fever, enteric fever, typhus abdominalis. |
Salmonella typhi |
Worldwide.,major diseases of which paratyphoid is the milder. |
A01.0,A01.4
No of cases annually estimated at 17 million cases annually with estimated 600,000! Deaths. Many mild and inapparent infections occur |
General |
Achlorhydria, HIV infection, IN endemic areas disease is most common in children up to 19 years of age. |
Relative specific immunity follows infection with disease, unapparent infection or active immunisation |
A double vaccine is available, one part live and the other a coat polysaccharide (from paratyphus). They are not uniformly successful. |
Humans for typhoid, and paratyphoid. More rarely animals for paratyphoid. Some chronic carriers. |
Antibiotics but resistance is becoming an increasingly difficult problem. |
| Typhus fever, epidemic louse borne typhus fever |
Rickettsia prowazekii
A wide variety of systsemic symptoms with a specifically recognized (Brill-Zinser) disease occurring year after the primary attack. |
In colder areas where people may live in unsanitary conditions and are infested with lice. |
A75,Case fatality untreated varies from 10-40%. Mild infections can occur without eruptions especially in children and those partially immunized |
General |
None stated |
One attack gives lifelong immunity This is stated in earlier editions of the manual but not repeated in the 20th edition. |
None |
Humans and to a limited extent flying squirrels. The vector is the body louse. |
Antibiotic treatment, doxycycline, usually effective. |
| Disease
|
Infectious agent.
Target organ if any. |
Location |
ICD 10 entry.
Impact |
Susceptibility |
Special risk factors |
Immunity |
Vaccines |
Primary reservoir and vectors |
Comments, treatment |
| Typhus fever, epidemic flea borne typhus, murine typhus, shop typhus. |
Rickettsia typhi
Manifestations of disease similar to those associated with louse borne disease. |
Worldwide |
A75.22
Milder than the louse borne equivalent |
General |
None given |
One attack confers immunity. |
None |
Rats mice and probably other small mammals, vector infected rat fleas. |
Tetracyclines |
| Scrub typhus, tsutsugamushi disease. Miteborne typhus fever |
Orientia tsutsugamushi with many serotypes. and a wide variety of symptoms often dermal initially. |
Central and South East Asia |
A75.3
Case fatality rate untreated as high as 60% |
General |
Bigger problems with older people, occupational particularly military troops |
Prolonged immunity against the homologous strain. Unpredictable for heterologous challenge |
None successful |
Thrombiculid mites are the reservoir |
Tetracyclines |
| Yaws, Frambesia tropica. |
Treponema pallidum
Highly unpleasant skin disorders. |
A disease of children in moist tropical regions |
A66Rarely fatal but can be very disfiguring and maiming. |
No evidence of natural or racial resistance |
More frequent in male children |
Infection results in immunity and sometimes resistance to other pathogenic treponemes |
None |
Humans |
Pencillin |
| Yersiniosis, |
Yersinia enterocolitica, Y. pseudotuberculosis
Typically manifest as acute febrile diarrhoea with abdominal pain. |
World wide |
A04.6Complex pattern of susceptibility, post infection arthritis is more severe in adolescents and young adults |
No statement but inference is that susceptibility is universal |
HLa-B27 positive patients more susceptible to reactive arthritis and Reiters syndrome. Septicaemia occurs more often in those with an iron overload or with underlying immunosuppression. |
Nothing stated |
None |
Animals particularly the pig, ie a zoonosis |
Organisms are sensitive to many antibiotics but not to penicillin. |