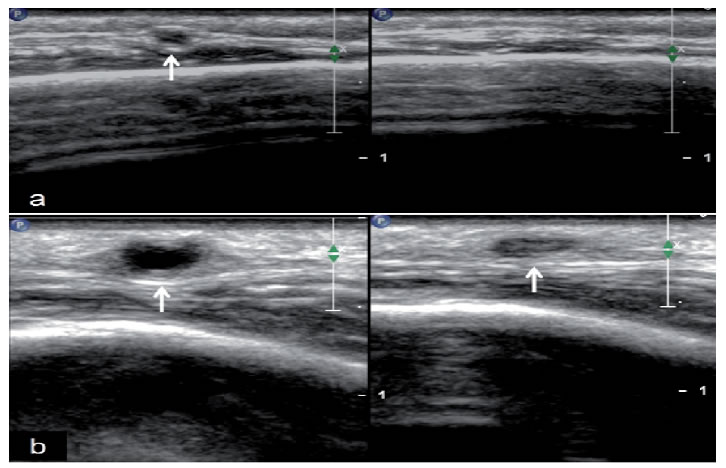
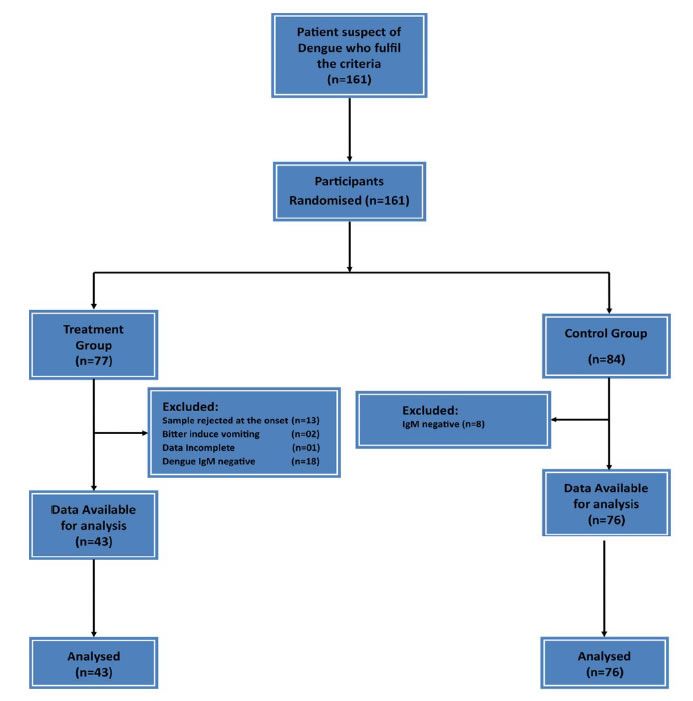
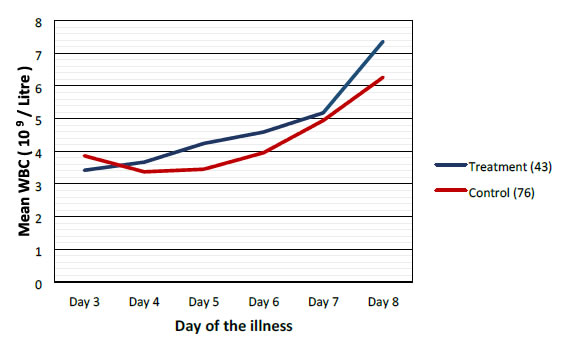
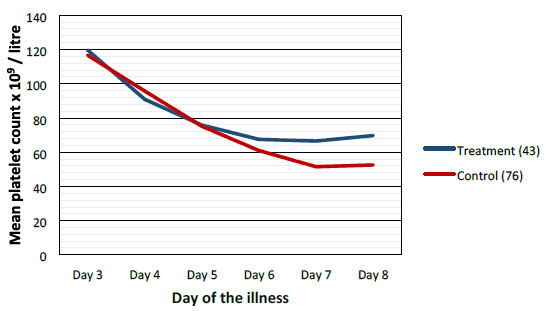
|
Appendix table. List of excluded papers
|
|
No.#
|
Authors
|
Title
|
Covid-19
Yes/No
|
Reason for exclusion
|
|
1
|
Khot WY, Nadkar MY. The 2019 Novel Coronavirus Outbreak – A Global Threat. J Assoc Physicians India. 2020;68:67-71.
|
The 2019 Novel Coronavirus Outbreak – A Global Threat
|
Yes
|
No details on LPV/r therapeutics
|
|
2
|
Ahmad A, Rehman MU, Alkharfy KM. An alternative approach to minimize the risk of coronavirus (Covid-19) and similar infections. Eur Rev Med Pharmacol Sci. 2020;24:4030-4.
|
An alternative approach to minimize the risk of coronavirus (Covid-19) and similar infections
|
Yes
|
No details on LPV/r therapeutics
|
|
3
|
Khan Z, Karatas Y, Rahman H. Anti COVID-19 Drugs: Need for More Clinical Evidence and Global Action. Adv Ther. 2020. Apr 29.
DOI: 10.1007/s12325-020-01351-9
|
Anti COVID-19 Drugs: Need for More Clinical Evidence and Global Action
|
Yes
|
Review
|
|
4
|
Yousefifard M, Zali A, Mohamed Ali K, Madani Neishaboori A, Zarghi A, Hosseini M, et al. Antiviral therapy in management of COVID-19: a systematic review on current evidence. Arch Acad Emerg Med. 2020;8:e45.
|
Antiviral therapy in management of COVID-19: a systematic review on current evidence.
|
Yes
|
Review
|
|
5
|
Simsek Yavuz S, Unal S. Antiviral treatment of COVID-19. Turk J Med Sci. 2020;50:611-9.
|
Antiviral treatment of COVID-19
|
Yes
|
Review
|
|
6
|
Vanden Eynde JJ. COVID-19: A Brief Overview of the Discovery Clinical Trial. Pharmaceuticals (Basel, Switzerland) 2020 Apr 10.
DOI: 10.3390/ph13040065
|
A Brief Overview of the Discovery Clinical Trial
|
Yes
|
Review
|
|
7
|
Liu YJ, Yang YL, Xu Y. [What we learned from SARS may provide important insights into understanding and management of coronavirus disease 2019]. Zhonghua Jie He He Hu Xi Za Zhi 2020 Apr 12;43:339-344.
|
[What we learned from SARS may provide important insights into understanding and management of coronavirus disease 2019]
|
Yes
|
Review
|
|
8
|
Rubin EJ, Baden LR, Morrissey S. Audio Interview: New Research on Possible Treatments for Covid-19. N Engl J Med. 2020;382:e30.
|
Audio Interview: New Research on Possible Treatments for Covid-19
|
Yes
|
Review
|
|
9
|
Lim J, Jeon S, Shin HY, Kim MJ, Seong YM, Lee WJ, et al. The Author’s Response: Case of the Index Patient Who Caused Tertiary Transmission of Coronavirus Disease 2019 in Korea: the Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Pneumonia Monitored by Quantitative RT-PCR. J Korean Med Sci. 2020;35:e89.
|
Author’s Response: Case of the Index Patient Who Caused Tertiary Transmission of Coronavirus Disease 2019 in Korea: the Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Pneumonia Monitored by Quantitative RT-PCR
|
Yes
|
No details on LPV/r therapeutics
|
|
10
|
Mothay D, Ramesh KV. Binding site analysis of potential protease inhibitors of COVID-19 using AutoDock. Virusdisease. 2020 2:1-6.
|
Binding site analysis of potential protease inhibitors of COVID-19 using AutoDock
|
Yes
|
No details on LPV/r therapeutics
|
|
11
|
McKee DL, Sternberg A, Stange U, Laufer S, Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res. 2020:104859.
|
Candidate drugs against SARS-CoV-2 and COVID-19
|
Yes
|
Review
|
|
12
|
Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020. Mar 27. DOI: 10.1001/jamacardio.2020.1096
|
Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19)
|
Yes
|
No details on LPV/r therapeutics
|
|
13
|
Naksuk N, Lazar S, Peeraphatdit TB. Cardiac safety of off-label COVID-19 drug therapy: a review and proposed monitoring protocol. Eur Heart J Acute Cardiovasc Care. 2020:2048872620922784.
|
Cardiac safety of off-label COVID-19 drug therapy: a review and proposed monitoring protocol
|
Yes
|
No details on LPV/r therapeutics
|
|
14
|
Kakodkar P, Kaka N, Baig MN. A Comprehensive Literature Review on the Clinical Presentation, and Management of the Pandemic Coronavirus Disease 2019 (COVID-19). Cureus 2020 Apr 06;12:1.
|
Comprehensive Literature Review on the Clinical Presentation, and Management of the Pandemic Coronavirus Disease 2019 (COVID-19)
|
Yes
|
Review
|
|
15
|
Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020. Mar 25.
DOI: 10.1016/S1473-3099(20)30198-5
|
Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study
|
Yes
|
No details on LPV/r therapeutics
|
|
16
|
Allameh S.F. All about COVID-19 in brief. New Microbes and New Infections. 2020;35:no pagination.
|
All about COVID-19 in brief
|
Yes
|
No details on LPV/r therapeutics
|
|
17
|
Du B, Qiu HB, Zhan X, Wang YS, Kang HYJ, Li XY, et al. [Pharmacotherapeutics for the new coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi 2020 Mar 12;43:173-176.
|
[Pharmacotherapeutics for the new coronavirus pneumonia]
|
Yes
|
Review
|
|
18
|
Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect. 2020. Mar 27.
DOI: 10.1016/j.jinf.2020.03.005
|
Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients
|
Yes
|
No details on LPV/r therapeutics
|
|
19
|
Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical Features of COVID-19-Related Liver Damage. Clin Gastroenterol Hepatol. 2020. Apr 10.
DOI: 10.1016/j.cgh.2020.04.002.
|
Clinical Features of COVID-19-Related Liver Damage
|
Yes
|
No details on LPV/r therapeutics
|
|
20
|
Rosa SGV, Santos WC. Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Publica. 2020;44:e40.
|
Clinical trials on drug repositioning for COVID-19 treatment
|
Yes
|
Review
|
|
21
|
Martinez MA. Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus. Antimicrob Agents Chemother. 2020;Apr 21.
DOI: 10.1128/AAC.00399-20
|
Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus
|
Yes
|
Review
|
|
22
|
Lv DF, Ying QM, Weng YS, Shen CB, Chu JG, Kong JP, et al. Dynamic change process of target genes by RT-PCR testing of SARS-Cov-2 during the course of a Coronavirus Disease 2019 patient. Clin Chim Acta. 2020;506:172-5.
|
Dynamic change process of target genes by RT-PCR testing of SARS-Cov-2 during the course of a Coronavirus Disease 2019 patient
|
Yes
|
No details on LPV/r therapeutics
|
|
23
|
Muralidharan N, Sakthivel R, Velmurugan D, Gromiha MM. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 protease against COVID-19. J Biomol Struct Dyn. 2020; 16:1-6.
|
Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 protease against COVID-19
|
Yes
|
No details on LPV/r therapeutics
|
|
24
|
Song J, Kang S, Choi SW, Seo KW, Lee S, So MW, et al. Coronavirus Disease 19 (COVID-19) complicated with pneumonia in a patient with rheumatoid arthritis receiving conventional disease-modifying antirheumatic drugs. Rheumatol Int. 2020;40:991-5.
|
Coronavirus Disease 19 (COVID-19) complicated with pneumonia in a patient with rheumatoid arthritis receiving conventional disease-modifying antirheumatic drugs
|
Yes
|
No details on LPV/r therapeutics
|
|
25
|
Wang M, Zhou Y, Zong Z, Liang Z, Cao Y, Tang H, et al. A precision medicine approach to managing 2019 novel coronavirus pneumonia. Precis Clin Med. 2020;3:14-21.
|
A precision medicine approach to managing 2019 novel coronavirus pneumonia
|
Yes
|
No details on LPV/r therapeutics
|
|
26
|
McCreary EK, Pogue JM. Coronavirus Disease 2019 Treatment: A Review of Early and Emerging Options. Open Forum Infect Dis. 2020;7:ofaa105.
|
Coronavirus Disease 2019 Treatment: A Review of Early and Emerging Options
|
Yes
|
Review
|
|
27
|
Yuan J, Zou R, Zeng L, Kou S, Lan J, Li X, et al. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm Res. 2020;69:599-606.
|
The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients
|
Yes
|
No details on LPV/r therapeutics
|
|
28
|
Han W, Quan B, Guo Y, Zhang J, Lu Y, Feng G, et al. The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019. J Med Virol. 2020;92:461-3.
|
The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019
|
Yes
|
No details on LPV/r therapeutics
|
|
29
|
Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020. Apr 30.
DOI:10.1093/cvr/cvaa106
|
COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment option
|
Yes
|
Review
|
|
30
|
Sankar J, Dhochak N, Kabra SK, Lodha R. COVID-19 in Children: Clinical Approach and Management. Indian J Pediatr. 2020. Apr 27.
DOI: 10.1007/s12098-020-03292-1
|
COVID-19 in Children: Clinical Approach and Management
|
Yes
|
Review
|
|
31
|
Ma J, Xia P, Zhou Y, Liu Z, Zhou X, Wang J, et al. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin Immunol. 2020;214:108408.
|
Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19
|
Yes
|
No details on LPV/r therapeutics
|
|
32
|
Bleasel MD, Peterson GM. Emetine, Ipecac, Ipecac Alkaloids and Analogues as Potential Antiviral Agents for Coronaviruses. Pharmaceuticals (Basel). 2020; Mar 21.
DOI: 10.3390/ph13030051.
|
Emetine, Ipecac, Ipecac Alkaloids and Analogues as Potential Antiviral Agents for Coronaviruses
|
Yes
|
Review
|
|
33
|
Arshad Ali S, Baloch M, Ahmed N, Arshad Ali A, Iqbal A. The outbreak of Coronavirus Disease 2019 (COVID-19)-An emerging global health threat. J Infect Public Health. 2020;13:644-6.
|
The outbreak of Coronavirus Disease 2019 (COVID-19)-An emerging global health threat
|
Yes
|
No details on LPV/r therapeutics
|
|
34
|
Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID-19: Abnormal liver function tests. J Hepatol. 2020. Apr 13.
DOI: 10.1016/j.jhep.2020.04.006
|
COVID-19: Abnormal liver function tests
|
Yes
|
No details on LPV/r therapeutics
|
|
35
|
Chan KW, Wong VT, Tang SCW. COVID-19: An Update on the Epidemiological, Clinical, Preventive and Therapeutic Evidence and Guidelines of Integrative Chinese-Western Medicine for the Management of 2019 Novel Coronavirus Disease. Am J Chin Med. 2020. Mar 13.
DOI: 10.1142/S0192415X20500378
|
Update on the Epidemiological, Clinical, Preventive and Therapeutic Evidence and Guidelines of Integrative Chinese-Western Medicine for the Management of 2019 Novel Coronavirus Disease
|
Yes
|
Review
|
|
36
|
Scavone C, Brusco S, Bertini M, Sportiello L, Rafaniello C, Zoccoli A, et al. Current pharmacological treatments for COVID-19: what’s next? Br J Pharmacol. 2020.Apr 24.
DOI:10.1111/bph.15072
|
Current pharmacological treatments for COVID-19: what’s next?
|
Yes
|
Review
|
|
37
|
Tursen U, Tursen B, Lotti T. Cutaneous Side-Effects of the Potential Covid-19 Drugs. Dermatol Ther. 2020. May 5.
DOI: 10.1111/dth.13476
|
Cutaneous Side-Effects of the Potential Covid-19 Drugs
|
Yes
|
Review
|
|
38
|
Testa S, Prandoni P, Paoletti O, Morandini R, Tala M, Dellanoce C, et al. Direct oral anticoagulant plasma levels’ striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral agents: The Cremona experience. J Thromb Haemost. 2020. Apr 23.
DOI: 10.1111/jth.14871
|
Direct oral anticoagulant plasma levels’ striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral
|
Yes
|
No details on LPV/r therapeutics
|
|
39
|
Wu F, Zhang W, Zhang L, Wang D, Wan Y. Discontinuation of antiviral drugs may be the reason for recovered COVID-19 patients testing positive again. Br J Hosp Med (Lond). 2020;81:1-2.
|
Discontinuation of antiviral drugs may be the reason for recovered COVID-19 patients testing positive again
|
Yes
|
No details on LPV/r therapeutics
|
|
40
|
Zheng XW, Tao G, Zhang YW, Yang GN, Huang P. [Drug interaction monitoring of lopinavir / ritonavir in COVID-19 patients with cancer]. Zhonghua Nei Ke Za Zhi. 2020;59:E004.
|
[Drug interaction monitoring of lopinavir / ritonavir in COVID-19 patients with cancer]
|
Yes
|
Review
|
|
41
|
Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends. 2020;14:69-71.
|
Drug treatment options for the 2019-new coronavirus (2019-nCoV)
|
Yes
|
Review
|
|
42
|
Holzhauser L, Lourenco L, Sarswat N, Kim G, Chung B, Nguyen AB. Early Experience of COVID-19 in Two Heart Transplant Recipients: Case Reports and Review of Treatment Options. Am J Transplant. 2020. May 7.
DOI: 10.1111/ajt.15982.
|
Early Experience of COVID-19 in Two Heart Transplant Recipients: Case Reports and Review of Treatment Options
|
Yes
|
Review
|
|
43
|
Diurno F, Numis FG, Porta G, Cirillo F, Maddaluno S, Ragozzino A, et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24:4040-7.
|
Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience
|
Yes
|
No details on LPV/r therapeutics
|
|
44
|
Zhong H, Wang Y, Zhang ZL, Liu YX, Le KJ, Cui M, et al. Efficacy and safety of current therapeutic options for COVID-19 – lessons to be learnt from SARS and MERS epidemic: A systematic review and meta-analysis. Pharmacol Res. 2020. Apr 30.
DOI:10.1016/j.phrs.2020.104872
|
Efficacy and safety of current therapeutic options for COVID-19 – lessons to be learnt from SARS and MERS epidemic: A systematic review and meta-analysis
|
Yes
|
Review
|
|
45
|
Zhu S, Guo X, Geary K, Zhang D. Emerging Therapeutic Strategies for COVID-19 patients. Discoveries (Craiova). 2020;8:e105.
|
Emerging Therapeutic Strategies for COVID-19 patients.
|
Yes
|
Review
|
|
46
|
Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing). 2020. Mar 18.
DOI: 10.1016/j.eng.2020.03.007
|
Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study
|
Yes
|
No details on LPV/r therapeutics
|
|
47
|
Wang J. Fast Identification of Possible Drug Treatment of Coronavirus Disease-19 (COVID-19) through Computational Drug Repurposing Study. J Chem Inf Model. 2020. May 4.
DOI: 10.1021/acs.jcim.0c00179
|
Fast Identification of Possible Drug Treatment of Coronavirus Disease-19 (COVID-19) through Computational Drug Repurposing Study
|
Yes
|
No details on LPV/r therapeutics
|
|
48
|
Du YX, Chen XP. Favipiravir: Pharmacokinetics and Concerns About Clinical Trials for 2019-nCoV Infection. Clin Pharmacol Ther. 2020. Apr 4.
DOI: 10.1002/cpt.1844.
|
Favipiravir: Pharmacokinetics and Concerns About Clinical Trials for 2019-nCoV Infection
|
Yes
|
Review
|
|
49
|
Mullard A. Flooded by the torrent: the COVID-19 drug pipeline. Lancet. 2020;395:1245-6.
|
Flooded by the torrent: the COVID-19 drug pipeline
|
Yes
|
Review
|
|
50
|
Sapp JL, Alqarawi W, MacIntyre CJ, Tadros R, Steinberg C, Roberts JD, et al. Guidance on Minimizing Risk of Drug-Induced Ventricular Arrhythmia During Treatment of COVID-19: A Statement from the Canadian Heart Rhythm Society. Can J Cardiol. 2020. Apr 8.
DOI: 10.1016/j.cjca.2020.04.003
|
Guidance on Minimizing Risk of Drug-Induced Ventricular Arrhythmia During Treatment of COVID-19: A Statement from the Canadian Heart Rhythm Society
|
Yes
|
Guidelines
|
|
51
|
Sun J, Deng X, Chen X, Huang J, Huang S, Li Y, et al. Incidence of Adverse Drug Reactions in COVID-19 patients in China: an active monitoring study by Hospital Pharmacovigilance System. Clin Pharmacol Ther. 2020. Apr 23.
DOI: 10.1002/cpt.1866
|
Incidence of Adverse Drug Reactions in COVID-19 patients in China: an active monitoring study by Hospital Pharmacovigilance System
|
Yes
|
Review
|
|
52
|
Paital B, Das K, Parida SK. Inter nation social lockdown versus medical care against COVID-19, a mild environmental insight with special reference to India. Sci Total Environ. 2020;728:138914.
|
Inter nation social lockdown versus medical care against COVID-19, a mild environmental insight with special reference to India
|
Yes
|
No details on LPV/r therapeutics
|
|
53
|
Kim JY. Letter to the Editor: Case of the Index Patient Who Caused Tertiary Transmission of Coronavirus Disease 2019 in Korea: the Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Pneumonia Monitored by Quantitative RT-PCR. J Korean Med Sci. 2020;35:e88.
|
Letter to the Editor: Case of the Index Patient Who Caused Tertiary Transmission of Coronavirus Disease 2019 in Korea: the Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Pneumonia Monitored by Quantitative RT-PCR
|
Yes
|
Review
|
|
54
|
Rubel AR, Chong PL, Abdullah MS, Asli R, Momin RN, Mani BI, et al. Letter to the Editor: Lipemic serum in patients with COVID-19 undergoing treatment. J Med Virol. 2020. Apr 28.
DOI: 10.1002/jmv.25942
|
Letter to the Editor: Lipemic serum in patients with COVID-19 undergoing treatment
|
Yes
|
No details on LPV/r therapeutics
|
|
55
|
Stower H. Lopinavir-ritonavir in severe COVID-19. Nat Med. 2020;26:465.
|
Lopinavir-ritonavir in severe COVID-19
|
Yes
|
No details on LPV/r therapeutics
|
|
56
|
Bhatnagar T, Murhekar MV, Soneja M, Gupta N, Giri S, Wig N, et al. Lopinavir/ritonavir combination therapy amongst symptomatic coronavirus disease 2019 patients in India: Protocol for restricted public health emergency use. Indian J Med Res. 2020;151:184-9.
|
Lopinavir/ritonavir combination therapy amongst symptomatic coronavirus disease 2019 patients in India: Protocol for restricted public health emergency use
|
Yes
|
No details on LPV/r therapeutics
|
|
57
|
Docea AO, Tsatsakis A, Albulescu D, Cristea O, Zlatian O, Vinceti M, et al. A new threat from an old enemy: Reemergence of coronavirus (Review). Int J Mol Med. 2020;45:1631-43.
|
A new threat from an old enemy: Reemergence of coronavirus (Review)
|
Yes
|
Review
|
|
58
|
Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther. 2020;14:58-60.
|
Discovering drugs to treat coronavirus disease 2019 (COVID-19)
|
Yes
|
Review
|
|
59
|
Xu K, Cai H, Shen Y, Ni Q, Chen Y, Hu S, et al. [Management of corona virus disease-19 (COVID-19): the Zhejiang experience]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:0.
|
[Management of corona virus disease-19 (COVID-19): the Zhejiang experience]
|
Yes
|
Review
|
|
60
|
Lenkens M, de Wit H, Danser AH, Esselink AC, Horikx A, Ten Oever J, et al. [Medication and comedication in COVID-19 patients]. Ned Tijdschr Geneeskd. 2020;164.
|
[Medication and comedication in COVID-19 patients].
|
Yes
|
Review
|
|
61
|
Zhang P, Cai Z, Wu W, Peng L, Li Y, Chen C, et al. The novel coronavirus (COVID-19) pneumonia with negative detection of viral ribonucleic acid from nasopharyngeal swabs: a case report. BMC Infect Dis. 2020;20:317.
|
The novel coronavirus (COVID-19) pneumonia with negative detection of viral ribonucleic acid from nasopharyngeal swabs: a case report
|
Yes
|
No details on LPV/r therapeutics
|
|
62
|
Plusa T. [Options for controlling new Corona virus infection – 2019-nCoV]. Pol Merkur Lekarski. 2020;48:112-9.
|
[Options for controlling new Corona virus infection – 2019-nCoV]
|
Yes
|
Review
|
|
63
|
Pavone P, Ceccarelli M, Taibi R, La Rocca G, Nunnari G. Outbreak of COVID-19 infection in children: fear and serenity. Eur Rev Med Pharmacol Sci. 2020;24:4572-5.
|
Outbreak of COVID-19 infection in children: fear and serenity
|
Yes
|
Review
|
|
64
|
Yethindra V. Role of GS-5734 (Remdesivir) in inhibiting SARS-CoV and MERS-CoV: The expected role of GS-5734 (Remdesivir) in COVID-19 (2019-nCoV)-VYTR hypothesis. International Journal of Research in Pharmaceutical Sciences. 2020 Mar 6;11:1-6.
|
Role of GS-5734 (Remdesivir) in inhibiting SARS-CoV and MERS-CoV: The expected role of GS-5734 (Remdesivir) in COVID-19 (2019-nCoV)-VYTR hypothesis
|
Yes
|
No details on LPV/r therapeutics
|
|
65
|
Md Insiat Islam R. Current Drugs with Potential for Treatment of COVID-19: A Literature Review. J Pharm Pharm Sci. 2020;23:58-64.
|
Current Drugs with Potential for Treatment of COVID-19: A Literature Review
|
Yes
|
Review
|
|
66
|
Pant S, Singh M, Ravichandiran V, Murty USN, Srivastava HK. Peptide-like and small-molecule inhibitors against Covid-19. J Biomol Struct Dyn. 2020. May 6.
DOI: 10.1080/07391102.2020.1757510
|
Peptide-like and small-molecule inhibitors against Covid-19
|
Yes
|
No details on LPV/r therapeutics
|
|
67
|
Gupta R, Ghosh A, Singh AK, Misra A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab Syndr. 2020;14:211-2.
|
Clinical considerations for patients with diabetes in times of COVID-19 epidemic
|
Yes
|
No details on LPV/r therapeutics
|
|
68
|
Wei J, Xu H, Xiong J, Shen Q, Fan B, Ye C, et al. 2019 Novel Coronavirus (COVID-19) Pneumonia: Serial Computed Tomography Findings. Korean J Radiol. 2020;21:501-4.
|
2019 Novel Coronavirus (COVID-19) Pneumonia: Serial Computed Tomography Findings
|
Yes
|
No details on LPV/r therapeutics
|
|
69
|
Li H, Wang YM, Xu JY, Cao B. [Potential antiviral therapeutics for 2019 Novel Coronavirus]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:170-2.
|
[Potential antiviral therapeutics for 2019 Novel Coronavirus]
|
Yes
|
Review
|
|
70
|
Gyebi GA, Ogunro OB, Adegunloye AP, Ogunyemi OM, Afolabi SO. Potential Inhibitors of Coronavirus 3-Chymotrypsin-Like Protease (3CL(pro)): An in silico screening of Alkaloids and Terpenoids from African medicinal plants. J Biomol Struct Dyn. 2020. May 5.
DOI:10.1080/07391102.2020.1764868.
|
Potential Inhibitors of Coronavirus 3-Chymotrypsin-Like Protease (3CL(pro)): An in silico screening of Alkaloids and Terpenoids from African medicinal plants
|
Yes
|
No details on LPV/r therapeutics
|
|
71
|
Lu CC, Chen MY, Chang YL. Potential therapeutic agents against COVID-19: What we know so far. J Chin Med Assoc. 2020. Apr 1.
DOI:10.1097/JCMA.0000000000000318.
|
Potential therapeutic agents against COVID-19: What we know so far
|
Yes
|
Review
|
|
72
|
Beck BR, Shin B, Choi Y, Park S, Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput Struct Biotechnol J. 2020;18:784-90.
|
Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model
|
Yes
|
No details on LPV/r therapeutics
|
|
73
|
Gentile D, Patamia V, Scala A, Sciortino MT, Piperno A, Rescifina A. Putative Inhibitors of SARS-CoV-2 Main Protease from A Library of Marine Natural Products: A Virtual Screening and Molecular Modeling Study. Mar Drugs. 2020. Apr 23.
DOI: 10.3390/md18040225
|
Inhibitors of SARS-CoV-2 Main Protease from A Library of Marine Natural Products: A Virtual Screening and Molecular Modeling Study
|
Yes
|
No details on LPV/r therapeutics
|
|
74
|
Zhang Y, Xu J, Li H, Cao B. A Novel Coronavirus (COVID-19) Outbreak: A Call for Action. Chest. 2020;157:e99-e101.
|
A Novel Coronavirus (COVID-19) Outbreak: A Call for Action
|
Yes
|
Review
|
|
75
|
Kupferschmidt K, Cohen J. Race to find COVID-19 treatments accelerates. Science. 2020;367:1412-3.
|
Race to find COVID-19 treatments accelerates
|
Yes
|
Review
|
|
76
|
Choy KT, Wong AY, Kaewpreedee P, Sia SF, Chen D, Hui KPY, et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178:104786.
|
Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro
|
Yes
|
No details on LPV/r therapeutics
|
|
77
|
Kumar S, Zhi K, Mukherji A, Gerth K. Repurposing Antiviral Protease Inhibitors Using Extracellular Vesicles for Potential Therapy of COVID-19. Viruses. 2020. Apr 26.
DOI: 10.3390/v12050486
|
Repurposing Antiviral Protease Inhibitors Using Extracellular Vesicles for Potential Therapy of COVID-19
|
Yes
|
Review
|
|
78
|
Misra DP, Agarwal V, Gasparyan AY, Zimba O. Rheumatologists’ perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol. 2020. Apr 10.
DOI: 10.1007/s10067-020-05073-9
|
Rheumatologists’ perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets
|
Yes
|
Review
|
|
79
|
Xu X, Ong YK, Wang Y. Role of adjunctive treatment strategies in COVID-19 and a review of international and national clinical guidelines. Mil Med Res. 2020;7:22.
|
Role of adjunctive treatment strategies in COVID-19 and a review of international and national clinical guidelines
|
Yes
|
Review
|
|
80
|
Costanzo M, De Giglio MAR, Roviello GN. SARS-CoV-2: Recent Reports on Antiviral Therapies Based on Lopinavir/Ritonavir, Darunavir/Umifenovir, Hydroxychloroquine, Remdesivir, Favipiravir and Other Drugs for the Treatment of the New Coronavirus. Curr Med Chem. 2020. Apr 16
DOI:10.2174/0929867327666200416131117
|
SARS-CoV-2: Recent Reports on Antiviral Therapies Based on Lopinavir/Ritonavir, Darunavir/Umifenovir, Hydroxychloroquine, Remdesivir, Favipiravir and Other Drugs for the Treatment of the New Coronavirus
|
Yes
|
Review
|
|
81
|
Meziyerh S, Zwart TC, van Etten RW, Janson JA, van Gelder T, Alwayn IPJ, et al. Severe COVID-19 in a renal transplant recipient: A focus on pharmacokinetics. Am J Transplant. 2020. Apr 26.
DOI: 10.1111/ajt.15943
|
Severe COVID-19 in a renal transplant recipient: A focus on pharmacokinetics
|
Yes
|
No details on LPV/r therapeutics
|
|
82
|
Nham E, Ko JH, Jeong BH, Huh K, Cho SY, Kang CI, et al. Severe Thrombocytopenia in a Patient with COVID-19. Infect Chemother. 2020.
|
Severe Thrombocytopenia in a Patient with COVID-19
|
Yes
|
No details on LPV/r therapeutics
|
|
83
|
Unknown Author. Some drugs for COVID-19. Med Lett Drugs Ther. 2020;62:49-50.
|
Some drugs for COVID-19
|
Yes
|
Review
|
|
84
|
Nakamura K, Hikone M, Shimizu H, Kuwahara Y, Tanabe M, Kobayashi M, et al. A sporadic COVID-19 pneumonia treated with extracorporeal membrane oxygenation in Tokyo, Japan: A case report. J Infect Chemother. 2020. Apr 18.
DOI: 10.1016/j.jiac.2020.03.018
|
A sporadic COVID-19 pneumonia treated with extracorporeal membrane oxygenation in Tokyo, Japan: A case report
|
Yes
|
No details on LPV/r therapeutics
|
|
85
|
Yao TT, Qian JD, Zhu WY, Wang Y, Wang GQ. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option. J Med Virol. 2020. Feb 27.
DOI: 10.1002/jmv.25729.
|
A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option
|
Yes
|
Review
|
|
86
|
Ford N, Vitoria M, Rangaraj A, Norris SL, Calmy A, Doherty M. Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID-19: initial assessment. J Int AIDS Soc. 2020;23:e25489.
|
Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID-19: initial assessment
|
Yes
|
Review
|
|
87
|
Baron SA, Devaux C, Colson P, Raoult D, Rolain JM. Teicoplanin: an alternative drug for the treatment of COVID-19? Int J Antimicrob Agents. 2020;55:105944.
|
Teicoplanin: an alternative drug for the treatment of COVID-19?
|
Yes
|
Review
|
|
88
|
Bartiromo M, Borchi B, Botta A, Bagala A, Lugli G, Tilli M, et al. Threatening drug-drug interaction in a kidney transplant patient with Coronavirus Disease 2019 (COVID-19). Transpl Infect Dis. 2020. Apr 12.
DOI: 10.1111/tid.13286
|
Threatening drug-drug interaction in a kidney transplant patient with Coronavirus Disease 2019
|
Yes
|
No details on LPV/r therapeutics
|
|
89
|
Zhang H, Xie C, Huang Y. Treatment and Outcome of a Patient With Lung Cancer Infected With Severe Acute Respiratory Syndrome Coronavirus-2. J Thorac Oncol. 2020;15:e63-e4.
|
Treatment and Outcome of a Patient With Lung Cancer Infected With Severe Acute Respiratory Syndrome Coronavirus-2
|
Yes
|
No details on LPV/r therapeutics
|
|
90
|
Jean SS, Lee PI, Hsueh PR. Treatment options for COVID-19: The reality and challenges. J Microbiol Immunol Infect. 2020. Apr 4.
DOI: 10.1016/j.jmii.2020.03.034
|
Treatment options for COVID-19: The reality and challenges
|
Yes
|
Review
|
|
91
|
Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400-2.
|
COVID-19: combining antiviral and anti-inflammatory treatments
|
Yes
|
No details on LPV/r therapeutics
|
|
92
|
Carmona-Bayonas A, Jimenez-Fonseca P, Castanon E. A Trial of Lopinavir-Ritonavir in Covid-19. N Engl J Med. 2020. May 5
DOI:10.1056/NEJMc2008043
|
A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19
|
Yes
|
Letter to the editor
|
|
93
|
Corrao S, Natoli G, Cacopardo B. A Trial of Lopinavir-Ritonavir in Covid-19. N Engl J Med. 2020. May 5.
DOI:10.1056/NEJMc2008043
|
A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19
|
Yes
|
Letter to the editor
|
|
94
|
Dalerba P, Levin B, Thompson JL. A Trial of Lopinavir-Ritonavir in Covid-19. N Engl J Med. 2020. May 5.
DOI:10.1056/NEJMc2008043
|
A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19
|
Yes
|
Letter to the editor
|
|
95
|
Havlichek D, Jr. A Trial of Lopinavir-Ritonavir in Covid-19. N Engl J Med. 2020. May 5.
DOI:10.1056/NEJMc2008043
|
A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19
|
Yes
|
Letter to the editor
|
|
96
|
Kunz KM. A Trial of Lopinavir-Ritonavir in Covid-19. N Engl J Med. 2020. May 5.
DOI:10.1056/NEJMc2008043
|
A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19
|
Yes
|
Letter to the editor
|
|
97
|
Shah B, Modi P, Sagar SR. In silico studies on therapeutic agents for COVID-19: Drug repurposing approach. Life Sci. 2020;252:117652.
|
In silico studies on therapeutic agents for COVID-19: Drug repurposing approach
|
Yes
|
No details on LPV/r therapeutics
|
|
98
|
Kim Y, Kwon O, Paek JH, Park WY, Jin K, Hyun M, et al. Two distinct cases with COVID-19 in kidney transplant recipients. Am J Transplant. 2020. Apr 26.
DOI: 10.1111/ajt.15947.
|
Two distinct cases with COVID-19 in kidney transplant recipients
|
Yes
|
|
|
99
|
Qiu L, Jiao R, Zhang A, Chen X, Ning Q, Fang F, et al. A Typical Case of Critically Ill Infant of Coronavirus Disease 2019 With Persistent Reduction of T Lymphocytes. Pediatr Infect Dis J. 2020. May 1.
DOI: 10.1097/INF.0000000000002720
|
A Typical Case of Critically Ill Infant of Coronavirus Disease 2019 With Persistent Reduction of T Lymphocytes
|
Yes
|
No details on LPV/r therapeutics
|
|
100
|
Giudicessi JR, Noseworthy PA, Friedman PA, Ackerman MJ. Urgent Guidance for Navigating and Circumventing the QTc-Prolonging and Torsadogenic Potential of Possible Pharmacotherapies for Coronavirus Disease 19 (COVID-19). Mayo Clin Proc. 2020. Apr 7.
DOI: 10.1016/j.mayocp.2020.03.024.
|
Urgent Guidance for Navigating and Circumventing the QTc-Prolonging and Torsadogenic Potential of Possible Pharmacotherapies for Coronavirus Disease 19 (COVID-19)
|
Yes
|
No details on LPV/r therapeutics
|
|
101
|
Taniguchi H, Ogawa F, Honzawa H, Yamaguchi K, Niida S, Shinohara M, et al. Veno-venous extracorporeal membrane oxygenation for severe pneumonia: COVID-19 case in Japan. Acute Med Surg. 2020;7:e509.
|
Veno-venous extracorporeal membrane oxygenation for severe pneumonia: COVID-19 case in Japan
|
Yes
|
No details on LPV/r therapeutics
|
|
102
|
Larreal Y. Pandemic of the new coronavirus SARSCoV-2 in Venezuela. [Spanish]. Investigacion Clinica (Venezuela). 2020;61:pp 1-3.
|
Pandemic of the new coronavirus SARSCoV-2 in Venezuela.
|
Yes
|
No details on LPV/r therapeutics
|
|
103
|
Nutho B, Mahalapbutr P, Hengphasatporn K, Pattaranggoon NC, Simanon N, Shigeta Y, et al. Why Are Lopinavir and Ritonavir Effective against the Newly Emerged Coronavirus 2019? Atomistic Insights into the Inhibitory Mechanisms. Biochemistry. 2020. Apr 24.
DOI: 10.1021/acs.biochem.0c00160.
|
Why Are Lopinavir and Ritonavir Effective against the Newly Emerged Coronavirus 2019? Atomistic Insights into the Inhibitory Mechanisms
|
Yes
|
Review
|
|
104
|
Ning L, Liu L, Li W, Liu H, Wang J, Yao Z, et al. Novel coronavirus (SARS-CoV-2) infection in a renal transplant recipient: Case report. Am J Transplant. 2020. Apr 10.
DOI: 10.1111/ajt.15897
|
Novel coronavirus (SARS-CoV-2) infection in a renal transplant recipient: Case report
|
Yes
|
No details on LPV/r therapeutics
|
|
105
|
Decaro N, Martella V, Saif LJ, Buonavoglia C. COVID-19 from veterinary medicine and one health perspectives: What animal coronaviruses have taught us. Res Vet Sci. 2020;131:21-3.
|
COVID-19 from veterinary medicine and one health perspectives: What animal coronaviruses have taught us
|
Yes
|
No details on LPV/r therapeutics
|
|
106
|
Ortega JT, Serrano ML, Pujol FH, Rangel HR. Unrevealing sequence and structural features of novel coronavirus using in silico approaches: The main protease as molecular target. Excli j. 2020;19:400-9.
|
Unrevealing sequence and structural features of novel coronavirus using in silico approaches: The main protease as molecular target
|
Yes
|
No details on LPV/r therapeutics
|
|
107
|
Das S, Sarmah S, Lyndem S, Singha Roy A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J Biomol Struct Dyn. 2020. May 2.
DOI: 10.1080/07391102.2020.1763201.
|
An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study
|
Yes
|
No details on LPV/r therapeutics
|
|
108
|
Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. Jama. 2020. Mar 3.
DOI: 10.1001/jama.2020.3204.
|
Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore
|
Yes
|
No details on LPV/r therapeutics
|
|
109
|
Alpern JD, Gertner E. Off-Label Therapies for COVID-19-Are We All In This Together? Clin Pharmacol Ther. 2020. Apr 20.
DOI: 10.1002/cpt.1862.
|
Off-Label Therapies for COVID-19-Are We All In This Together?
|
Yes
|
Review
|
|
110
|
Buonaguro FM, Puzanov I, Ascierto PA. Anti-IL6R role in treatment of COVID-19-related ARDS. J Transl Med. 2020;18:165.
|
Anti-IL6R role in treatment of COVID-19-related ARDS
|
Yes
|
No details on LPV/r therapeutics
|
|
111
|
Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18:164.
|
Why tocilizumab could be an effective treatment for severe COVID-19?
|
Yes
|
No details on LPV/r therapeutics
|
|
112
|
Calligari P, Bobone S, Ricci G, Bocedi A. Molecular Investigation of SARS-CoV-2 Proteins and Their Interactions with Antiviral Drugs. Viruses. 2020; Apr 14.
DOI: 10.3390/v12040445
|
Molecular Investigation of SARS-CoV-2 Proteins and Their Interactions with Antiviral Drugs
|
Yes
|
No details on LPV/r therapeutics
|